Cannabinoid receptors are found throughout the human central nervous system, and they regulate a wide variety of functions including appetite, pain sensation, mood, and memory. This 3D computer illustration depicts δ-9-tetrahydrocannabinol (THC), in green, binding to a cannabinoid receptor (purple). Image by Juan Gaertner/Science Source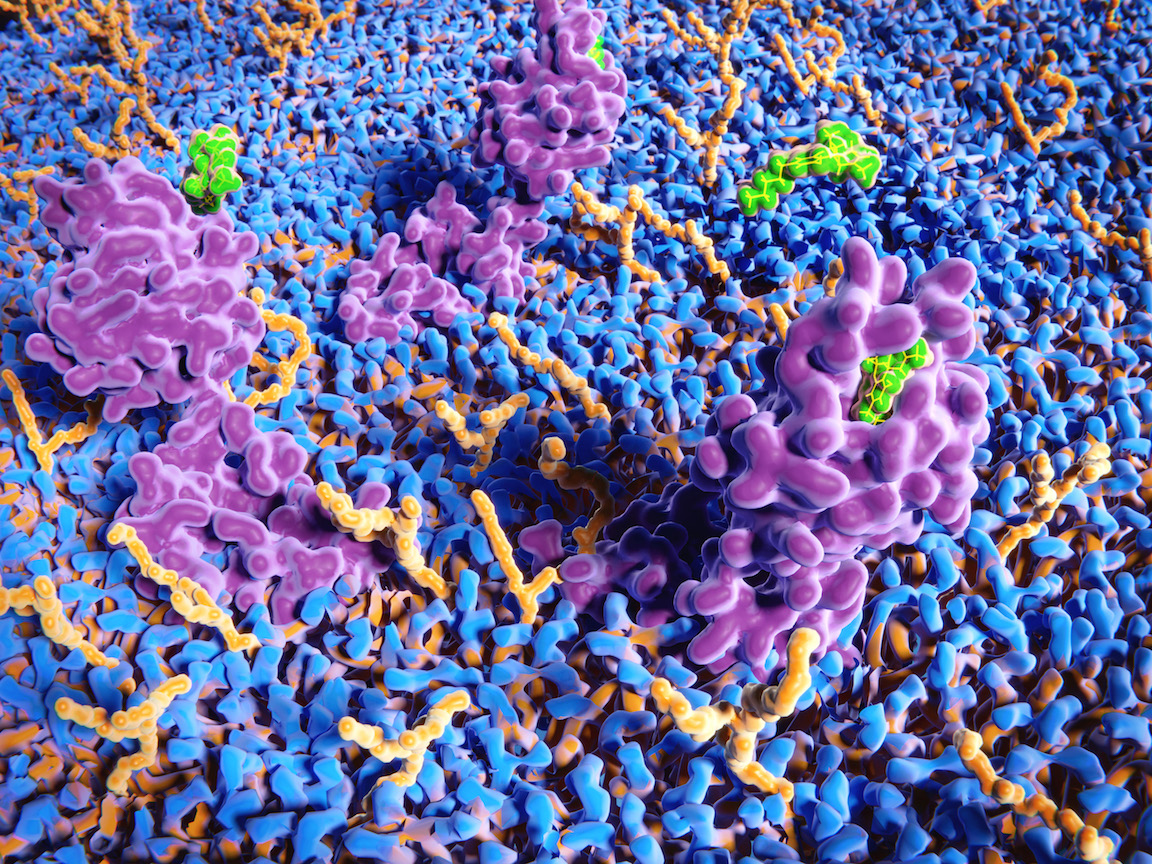
In addition, 11 states, and the District of Columbia, have legalized cannabis for recreational use by adults, with more states likely to follow.
These regulatory changes have been driven, in part, by a growing evidence base for the therapeutic use of phytocannabinoids, particularly cannabidiol (CBD), the non-intoxicating compound found in hemp and marijuana (both Cannabis Sativa).
Heightened scientific attention has been directed towards the mechanisms by which delta-9-tetrahydrocannabiniol (delta-9-THC), cannabidiol (CBD) and other phytocannabinoids exert their physiological effects. These exogenous, plant-derived ligands interact with endogenously produced proteins, receptors, enzymes and endogenous ligands, in one of the most evolutionarily preserved biological systems known to the life sciences: the endogenous cannabinoid signaling system, better known as the endocannabinoid system (ECS).
600 Million Years…and Counting
The ECS is thought to be 600 million years old. It is present in every animal species, except insects, and has evolved as a stress regulation network functioning to restore homeostasis following cellular stressors (McPartland, M. et al. Gene. 2006; 370: 64-74; Salahudeen, M. & Nishtala, P. Saudi Pharm J. 2017; 25(2): 165-175).
The discovery of the ECS began with Israeli researcher Raphael Mechoulam, and his team’s search for the “active ingredient” in cannabis. Decades earlier, CBD was first isolated from cannabis extract in 1940. Initially, it was deemed an inactive constituent because it failed to mimic the effects of cannabis extracts in animals and humans (Gaoni, Y. & Mechoulam, R. J Am Chem Soc. 1964; 86(8): 1646-1647). As a result, CBD was not characterized structurally until 1963, more than 20 years after it was first isolated.
Delta-9-THC was isolated and characterized in 1964, one year after CBD, and it did mimic the observable perceptual and behavioral effects of cannabis extracts. Consequently, THC became the main focus of phytocannabinoid research going forward. In addition to investigating its stereochemistry, pharmacokinetics, and other characteristics, scientists began to explore the pharmacology of delta-9-THC, specifically its mechanisms of action (Mechoulam, R. et al. Science. 1970; 169(3945): 611-612).
With the endogenous opioid system as their blueprint, researchers at St. Louis University discovered a G protein-coupled receptor to which delta-9-THC exhibited partial agonism. The discovery of this receptor (Cannabinoid receptor 1 or CB1) back in 1988 paved the way for the detection of endogenous ligands which demonstrated affinity for the receptor (Howlett, A. et al. Mol Pharmacol. 1988; 33(3): 297-302).
The endocannabinoid called arachidonoylethanolamine (AEA), better known as anandamide, was subsequently identified in 1992. A second endocannabinoid, called 2-arachidonoylglycerol (2-AG), was discovered in 1995.
In 1993, another cannabinoid receptor (CB2) was identified, mostly in the peripheral nervous system. Around this time, investigators became interested in the processes of production and breakdown of endocannabinoids. The enzymes responsible for synthesizing and degrading anandamide and 2-AG were identified in 1994 and 1993, respectively.
Endocannabinoids
Endocannabinoids are the signaling molecules of the endocannabinoid system. They are fatty acid neurotransmitters that coordinate and fine-tune intracellular biochemistry and intercellular communication across all physiological systems.
A multitude of pathological conditions may involve alterations, or disruptions, in endocannabinoid synthesis, degradation, receptor expression, or enzyme function (DiMarzo, V. & DePetrocellis, L. Philos Trans R Soc Lond B Biol Soc. 2012; 367(1607): 3216-3228).
The two most widely studied endocannabinoids are N-arachidonoylethanolamine (AEA) and 2-arachidonolyglycerol (2-AG).
N-arachidonoylethanolamine (AEA or Anandamide): Like most other lipid mediators, endocannabinoids have more than one set of biosynthetic and degrading pathways and enzymes (Di Marzo, V. & Piscitelli, F. Neurotherapeutics. 2015; 12(4): 692-698). Anandamide is predominantly synthesized by the enzyme N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD) and hydrolyzed intracellularly by fatty acid amide hydrolase (FAAH). It is a high affinity partial agonist of CB1 and CB2 receptors.
2-arachidonoylglycerol (2-AG): 2-AG is very similar to anandamide in chemical structure; however, it is a moderate affinity, full agonist for both CB1 and CB2 receptors. 2-AG is synthesized and hydrolyzed by different enzymes, namely diacylglycerol lipase (DAGL) and monoacylglycerol lipase (MAGL) respectively. 2-AG is the most abundant endocannabinoid in the central nervous system (CNS), and plays a major role in CNS development and synaptic plasticity.
ECS Receptors
Endocannabinoids mediate their effects primarily through interactions with receptors, both cannabinoid and non-cannabinoid receptors. Cannabinoid receptors are 7-transmembrane-domain G protein-coupled receptors. CB1 and CB2 differ in their amino acid sequence, anatomic distribution, mechanisms of signaling and other characteristics.
G-protein coupled receptors represent the most common receptor system in vertebrates, and CB1 receptors are the most abundant and densely concentrated receptors in the human central nervous system. Some have postulated that “cannabinoid receptor” may be a misnomer, given that THC and THC-V are the only phytocannabinoids that exhibit high affinity for binding domains on CB1 and CB2. Practically speaking, however, ligands that exhibit low affinity are still deemed cannabinoids. This includes CBD, which demonstrates negligible affinity for either CB1 or CB2 (Thomas, A. et al. Br J Pharmacol. 2007; 150(5): 613-623).
CB1 Receptors: The CB1 receptor is widely distributed throughout the nervous system, particularly in nociceptive areas of the brain and spinal cord, but also on certain cells of the immune system, adipose tissue, liver, muscle, reproductive cells, kidney and lungs.
These receptors are noticeably absent in the cardiac and respiratory centers of the brainstem, which is why cannabis does not depress respiration or stop the heart from beating. Respiratory depression, mediated by opioid receptors, is the most common cause of opioid overdose mortality; this is not a risk with phytocannabinoids.
CB2 Receptors: In contrast, CB2 receptors are mainly located in the periphery, on immune cells and lymphoid tissues, and in organs like the heart and liver (Galiegue, S. et al. Eur J Biochem. 1995; 232(1): 54-61). The pharmacodynamics of the CB2 receptor are different from those of CB1. When activated, both receptors inhibit adenylate cyclase, but unlike activation of CB1 receptors, activation of CB2 does not lead to hyperpolarization of neurons.
The predominance of CB2 receptors on immune cells and tissues underlies the immunomodulatory role of the ECS. This is in large part why one of the functional benefits of ECS activation is the downregulation of inflammation (Klein, TW. Nat Rev Immunol. 2005; 5(5): 400-411). Some researchers have theorized that activation of the CB2 receptor could provide an avenue for the treatment of various inflammatory conditions.
The ECS & Other Neurotransmitter Systems
While G protein-coupled receptors are common across most neurotransmitter systems (e.g., serotonergic, opioidergic, dopaminergic, etc.), other aspects of the ECS are quite unique.
For example, unlike most other neurotransmitters, endocannabinoids are lipids, not peptides like dopamine, GABA, acetylcholine, etc. In fact, endocannabinoids are the only lipid signaling molecules in the central nervous system.
Also, they are synthesized on demand, typically in post-ganglionic neurons, as opposed to being synthesized a priori and stored in synaptic vesicles at the terminal ends of pre-ganglionic neurons. And unlike other neurotransmitter systems, in which neurotransmitters diffuse in an anterograde direction from pre- to post-ganglionic neurons, the endocannabinoids diffuse in a retrograde direction, exerting their effects through receptors on pre-ganglionic neurons.
Finally, endocannabinoids are rapidly degraded, thus only exerting their effects for a short period of time.
Where Does CBD Fit In?
Unlike AEA, 2-AG and delta-9-THC, CBD does not act as a direct agonist of cannabinoid receptors. Instead, it is an allosteric modulator.
This means that it binds to a “non-active” site (i.e. the allosteric site). Allosteric modulators can prime receptors for potentiation or antagonism in subtle, yet powerful ways by changing the conformation of a receptor and influencing signal transduction. CBD has been referred to as a “functional antagonist” of CB1 receptors because it antagonizes delta-9-THC at the CB1 receptor sites, presumably through negative allosteric modulation (Tham, M. et al. Br J Pharmacol. 2019; 176(10): 1455-1469).
This is one of the ways in which CBD may attenuate some of the undesirable effects of delta-9-THC, such as anxiety, tachycardia and short-term memory loss (Russo, E. & Guy, GW. Medical Hypotheses. 2006; 66(2): 234-246). CBD may also act as a negative allosteric modulator of 2-AG.
CBD also acts as an agonist for a number of non-cannabinoid receptors, and it interacts with a wide variety of other molecular targets in the body, including enzymes and transport proteins. With more than 65 molecular targets identified, CBD is a highly promiscuous compound possessing a multitude of possible mechanisms of action.
CBD & Non-Cannabinoid Receptors
Allosteric modulators, like CBD, do not activate receptors directly. Thus CBD’s broad-reaching biological effects are largely attributable to other mechanisms, including interactions with non-cannabinoid receptors (Mechoulam, R. et al. J Clin Pharmacol. 2002; 42(S1): 11S-19S).
CBD interacts with opioid, serotonin, adenosine, and GABA receptors, as well as non-G protein coupled receptors like PPARγ and ligand-gated ion channels such as TRPV1.
This has clinical implications. One of the ways that CBD is thought to modulate inflammation, temperature and pain perception is through direct activation of TRPV1. Anti-anxiety and anti-emetic effects have been associated with direct and indirect agonism of the 5-HT1A receptor (de Mello Schier, AR. et al. CNS Neurol Disord Drug Targets. 2014; 13(6): 953-960).
CBD may also exert physiological influences through other indirect mechanisms. For example, it may affect intracellular signal transduction by disturbing neuronal membrane fluidity or by remodeling G-proteins associated with G-protein coupled receptors.
Receptor-Independent Mechanisms
CBD also appears to exert its effects through receptor-independent mechanisms. Interactions between phytocannabinoids and intracellular enzymes can inhibit enzymatic breakdown of endocannabinoids, thus increasing their signaling.
There is some evidence to suggest that CBD, like non-steroidal anti-inflammatory drugs (NSAIDS) can moderately inhibit anandamide hydrolysis in both mice and humans (De Petrocellis, L. et al. Br J Pharmacol. 2011; 163(7): 1479-1494), though another study indicates that it does not do so (Elmes, MW. et al. J Biol Chem. 2015; 290(14): 8711-8721).
Inhibition of Fatty Acid Binding Proteins (FABPs)
A family of intracellular transport proteins (Fatty Acid Binding Proteins or FABPs) effectively “solubilizes” cannabinoids and shuttles them from the cell membrane to the endoplasmic reticulum, where they are degraded by FAAH and monoacylglycerol lipase (MAGL).
There’s evidence that delta-9-THC and CBD compete with anandamide for binding sites on FABPs, thus inhibiting the cellular uptake and catabolism of anandamide. This competition may also explain increased circulating levels of anandamide reported after ingestion of CBD.
The finding came from an innovative study of CBD versus the antipsychotic drug, Amisulpride, in a cohort of 39 patients diagnosed with schizophrenia. The data suggested that use of CBD to inhibit the enzymatic degradation has antipsychotic effects, and represents “a completely new mechanism in the treatment of schizophrenia” (Leweke, FM. et al. Transl Psychiatry. 2012; 2(3): e94).
This mechanism may also partly explain the action of CBD in modulating endocannabinoid tone, and its effectiveness in treating epilepsy and other neurological disorders (Ibeas Bih, C. et al. Neurotherapeutics. 2015; 12(4): 699-730).
CBD for Pain & Inflammation
The ECS is upregulated and downregulated on a continuous basis as needed. It communicates with all other systems in the body and has been implicated in multiple regulatory functions in both health and disease, including pain, perception, mood, memory, reward and others. This vital physiological system is also affected by diet, sleep, exercise, stress, and a multitude of other factors, including exposure to phytocannabinoids.
Broadly speaking, cannabinoids are powerful modulators of inflammatory mediators. For example, CBD has been shown to inhibit tumor necrosis factor-alpha (TNF-α), and other inflammatory mediators in rodent models of acute pain and rheumatoid arthritis. Inhibition of FAAH and MAGL has been associated with increased endocannabinoid levels, analgesia, and opioid-sparing effects in animal models of pain (Wilkerson, JL. et al. Neuropharmacology. 2017; 114: 156-167).
Enhancement of adenosine signaling by CBD through inhibition of adenosine uptake has been associated with decreased inflammation in cell culture experiments (Carrier, EJ. et al. Proc Natl Acad Sci. 2006; 103(20): 7895-7900). Additionally, activation of TRPV1 has been shown to inhibit hyperalgesia in an animal model of acute pain. These mechanisms are not intended to represent an exhaustive list. Cannabinoid induced analgesia is likely the result of a complex interplay of mechanisms.
Tolerability & Safety of CBD
Comprehensive reviews of the safety and adverse effects of CBD conducted in 2011 and 2017 showed that even chronic use at very high doses (up to 1,500 mg/day of isolated CBD), is safe and well tolerated without significant adverse effects.
CBD does not appear to induce catalepsy, nor alter psychomotor and cognitive functions. In addition, the World Health Organization’s Expert Committee on Drug Dependence recommended that CBD should not be controlled by Schedule I of the 1961 UN Single Convention on Narcotic Drugs. Their updated comprehensive report is expected later this year.
All that said, CBD is not without adverse effects. The most commonly reported adverse effects are fatigue, diarrhea, dry mouth and changes of appetite/weight (Corroon, J. & Phillips, J. Cannabis Cannabinoid Research. 2018; 3(1): 152-161). Studies of patients with epilepsy and other seizure disorders indicate that extremely high doses (25 mg/kg or 50 mg/kg per day) of CBD have been associated with elevated liver enzymes in subjects taking multiple prescription drugs, including valproate.
The endocannabinoid system is an extensive endogenous signaling system. Our understanding of it is evolving rapidly.
The ECS is seemingly ubiquitous in animal species. It is affected by diet, sleep, exercise, stress and a multitude of other factors, including exposure to phytocannabinoids, like CBD and others. Actively modulating this system through targeted use of CBD and other phytocannabinoids may offer tremendous therapeutic promise for a diverse scope of diseases, including psychiatric conditions, neurological and movement disorders, pain, autoimmune disease, spinal cord injury, cancer, cardiometabolic disease, stroke, traumatic brain injury, osteoporosis, and others.
END
Jamie Corroon, ND, MPH, is the founder and Medical Director of the Center for Medical Cannabis Education, a clinic and educational resource center in Encinitas, CA. He earned his Doctor of Naturopathic Medicine degree from Bastyr University in Seattle, and completed two years of residency at the Bastyr Center for Natural Health. In addition to his ND degree, Dr. Corroon also holds a Master’s in Public Health in Epidemiology from San Diego State University. In addition to his clinical practice, Dr. Corroon is also a peer-reviewed clinical researcher, and industry consultant with a focus on medical Cannabis.
Jake Felice, ND, is a naturopathic physician in Seattle, WA. He specializes in the treatment of chronic pain and the improvement of human performance. He is a graduate of Bastyr University. In addition to his medical practice, he has served as a trainer for cannabis professionals in the Washington State Department of Health’s medical marijuana consultant certification program.