Liposomal formulations have been all the rage in the dietary supplement and nutraceutical industry over the last decade.
With their promises of better absorption, increased bioavailability, and improvements in efficacy, liposomal and “nanoparticle” formulas are popular with health-conscious consumers. They’re showing up with increasing frequency across the spectrum of natural products.
The “nanoparticle revolution” began with very real advances in chemistry. But as is the case with so many health trends, enthusiastic marketers jumped in, oversimplifying the science and obscuring the terminology as they tried to generate buzz.
It is a challenge to distinguish the real promise of these delivery systems from the marketing hype.
Basic Terminology
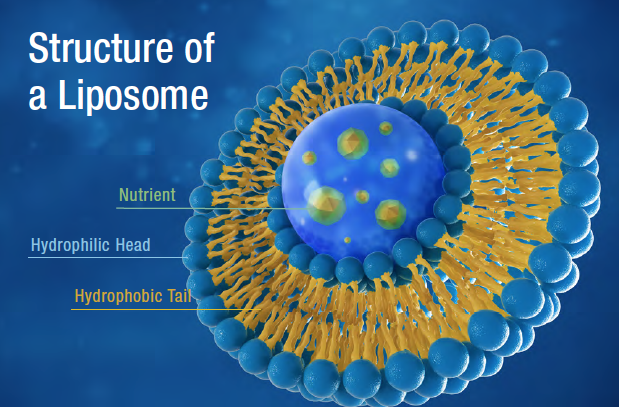
Technically, liposomes are a subset of lipid-based drug or nutrient delivery methods that utilize tiny phospholipid spheres to enapsulate water-soluble or lipid-soluble ingredients.
The phospholipid spheres may be single-layered, with their hydrophilic heads at the surface and their lipophilic tails toward the center. If these monolayered spheres contain nothing inside, they’re known as micelles. If the phospholipid monolayer contains an oil core of some sort, it is known as a nanoemulsion.
“Long-term stability is crucial and difficult to achieve in liposomes. Instability shows up most often in creaming, the rising to the top of free fats. On the other end of the spectrum, sedimentation is the falling out of insoluble compounds.”
Christopher Shade, PhD, Founder, Quicksilver Scientific
It is also possible to create bilayered phospholipid spheres that encapsulate water (and water-soluble ingredients). These are known as liposomes. The bilayers form concentrically: the outer layer has its hydrophilic heads at the surface with its tails toward the center, while the inner layer has its hydrophilic heads toward the center and its tails meeting those of the outer layer.
When properly made, the single and double-layered spheres should be “nanoparticles,” which means simply that the spheres are under 100 nanometers in diameter.
As a method for oral delivery of drugs or nutrients, nanoemulsions and liposomes have several advantages over standard tablet, capsule, or liquid forms, at least in principle. These include:
- Protection of bioactive ingredients from breakdown by stomach acid
- Higher bioavailability compared with other oral forms, leading to potential dose-sparing and improved cost efficiency
- Increased transmucosal and intestinal absorption and improved delivery of bioactive compounds to target tissues
- More adaptable to incremental dosing regimens
- Greater ease of use for people who have difficulty swallowing pills or capsules
“One of the great aspects of the sub-100 nm particles is that the Brownian movement of the particles—the random vibration inherent in all substances–exceeds the force of gravity that would naturally cause lipids and water to separate, with the oils going to the top,” explained Christopher Shade, PhD, CEO and founder of Quicksilver Scientific, one of the world’s leading producers and suppliers of liposomal and nanoemulsion-based nutraceuticals.
Liposomes, like other lipid particles, are cleared from the blood by the reticulo-endothelial system (RES), also called the mononuclear phagocyte system (MPS), a system of monocytes and macrophages in the lymph nodes, liver, and spleen. Shade says clearance correlates inversely with particle size: larger particles are cleared quickly; smaller ones remain in blood for longer periods.
Old Tech, New Applications
Liposomal and nanoemulsion technology is not new, Shade told Holistic Primary Care. It emerged as a potential drug delivery method back in the 1970s, and it is used in the production of many consumer products, from skin creams to foods, in which fats and water-based ingredients must be brought together in shelf-stable mixtures.
Long ago, food makers figured out how to use surfactants–molecules that can interact with both water and oil–to stabilize oil in water or vice versa. Soy-derived lecithin was among the first such surfactants, and it remains widely used. When synthetic surfactants, such as polyethylene glycols (PEGs) became available, emulsion technology grew in sophistication and breadth of application.
The technology took another evolutionary leap when high-shear processing methods, such as sonication and homogenization enabled producers to reduce the particle size of their emulsions down to the 10-200 nm range. By the late 1990s, liposomal formulations became the norm for many intravenous drugs including doxorubicin, amphotericin B, nystatin, and vincristine. Liposomes modify drug distribution in the body and mitigate somewhat the potential toxicities of these compounds.
It was only fairly recently that supplement companies became interested in liposomes. The shift was driven by research showing that many popular supplement ingredients like glutathione, B vitamins, Quercetin, Resveratrol, Berberine, Curcumin, Silymarin, and even Vitamins C and D, are not well-absorbed from standard oral tablets or capsules.
The Glutathione Conundrum
Dr. Shade, an environmental biochemist by training, says his interest in liposomes and nanoemulsions arose from a life-long concern about—and fascination with– mercury. He grew up in a Pennsylvania steel town where heavy metal pollution and mercury toxicity were all too common.
Quicksilver Scientific actually began as a testing company, providing medical practitioners with specialized tests for subspecies of mercury, based on technology that Shade patented while in graduate school.
“I was interested in the question of how different types of mercury are distributed in the body. That highlighted the problem of detoxification,” he said.
“I started thinking more deeply about how the body is supposed to deal with mercury, how to detoxify it. And that led me to focusing on glutathione.”
“I used myself as a laboratory. I got all my mercury amalgam (fillings) taken out, and then proceeded to do what most people did then which was to use synthetic chelators. I used DMSA (dimercaptosuccinic acid). The idea was to get all of the mercury out of my body. But it really wasn’t coming out of my body, it was just making me sicker and sicker. I started thinking more deeply about how the body is supposed to deal with mercury, how to detoxify it. And that led me to focusing on glutathione.”
Supplemental glutathione can be extremely helpful in detox protocols. But it is notoriously difficult to deliver to the target tissues. “Ordinary capsules don’t work for glutathione. There’s a bioavailability issue. The GI tract just takes apart the glutathione and you absorb the amino acids, not glutathione.”
In a search for ways to increase glutathione absorption, Shade explored nebulizer and transdermal delivery methods, which kindled an interest in liposomes.
He found a manufacturer who claimed to produce liposomal glutathione. “They sold me on the dream of liposomes, but the products didn’t deliver on the claims. They claimed to be at the nano range of under 100 nm. But I had the stuff analyzed. It was not “nano.”
Applying the old adage, “If you want something done right, do it yourself,” Shade invested in the technology to produce his own liposomal glutathione, as well as nanoemulsion forms of other low-absorption ingredients.
Smaller is Better
The advantages of liposomal and nanoemulsion formulations are predicated on increasing both absorption—the amount of the desired substance that passes from the GI lumen into the bloodstream–and bioavailability, the amount of the active compound reaching the target tissues. At least in principle, by increasing absorption and bioavailability, one increases bioactivity— the net physiological impact.
Shade says a lot of companies are playing loose and fast with these terms, and not really backing up their claims with analytical studies of their own formulas. Likewise, some claim their products to be “liposomal” or “nano” when in actuality they are not.
A “nano” or “liposomal” solution that appears cloudy, milky, or opaque contains particles that are 400 nm or greater, which puts them out of true nano range.
The improved absorption happens when the phospholipid spheres are under 100 nm in size. But some so-called liposomal formulas contain particles in the 400-1000 nm range.
In a lab setting, researchers use a method called Dynamic Light Scattering (DLS), also called photon correlation spectrometry to assess particle size. This makes use of the scatter patterns made by a laser beam as it passes through the liquid sample. Absolute particle size and shape are confirmed with electron microscopy. Shade says Quicksilver invested in 3 DLS machines to constantly test its own formulas.
Seek Transparency
“Long-term stability is crucial and difficult to achieve in liposomes. Instability shows up most often in creaming, the rising to the top of free fats. On the other end of the spectrum, sedimentation is the falling out of insoluble compounds.” Either of these indicates a deterioration of the emulsion and loss of the potential benefits.

Fortunately, there’s a simple no-cost way for practitioners and patients to get a reasonably accurate sense of whether a product is truly “nano” or not: transparency.
An emulsion with consistent particle size under 100 nm will appear completely transparent to the naked eye. That’s because the particles are smaller than the shortest wavelength of visible light, which is around 400 nm, so they do not block passage of light.

A “nano” or “liposomal” solution that appears cloudy, milky, or opaque contains particles that are 400 nm or greater, which puts them out of true nano range.
Stability of the emulsion and even distribution of the phosopholipid particles are also important quality variables, says Shade.
“This needs to be factored into the expiration date of these products. It’s not just the expiration of, say the glutathione itself, but also the life expectancy of the liposomes. Typically, this is in the range of 1-3 years.”
For some ingredients, the liposomes themselves last longer than the active compound inside them. This is true of glutathione, and nicotinamide mononucleotide (NMN), which only lasts for about a year.”
Shade says Quicksilver has invested heavily in its analytical testing capabilities.
Measuring Particle Size
“We are the only company that puts particle size range information for each batch on our certificates of analysis. We measure particle size first during the manufacturing process. Particle size is what determines stability, consistency, absorption, and efficacy.”
Quicksilver also runs routine pharmacokinetic analyses on its nanoemulsions, using classic “Area Under the Curve” methods that compare blood levels of the nanoemulsion formulations against curves tracing blood levels from intravenous dosing of the same compounds.

“We invested a quarter of a million dollars in a Liquid Chromatography/Mass Spectrometry triple quad system, which is a fancy way of finding a compound by measuring its mass in blood.”
There’s no question that true liposomal or nanoemulsion formulas will be more expensive than standard tablet or capsule formulations of the same ingredient.
If nanotechnology in the supplement industry is to deliver on its promises of better absorption and improved efficacy, and not devolve into an empty marketing gimmick, the companies producing these products must make the investments in production technology and product testing to ensure the integrity of their products.
Educated practitioners who understand the potential of liposomal and nanoemulsion formulas can play an important role in taking supplement brands to task on their “nano” claims.
END







