
The COVID-19 pandemic has been radically disruptive to all sectors of the economy, especially those connected with healthcare. The natural products and dietary supplements industries are no exception.
Since early March, when the industry’s 80,000-strong ExpoWest trade show was abruptly cancelled—the world’s supplement makers and the raw materials companies that supply them have rallied to meet the myriad challenges posed by the COVID crisis.
Those challenges are significant:
- A sudden surge in consumer demand for health-promoting products simultaneous with equally sudden shortages of key raw materials from China and other parts of Asia;
- Rising costs for shipping and longer wait-times on imported materials;
- New social distancing and employee-protection requirements;
- Increased scrutiny and enforcement actions from federal agencies working to protect the public from fraudulent COVID “cures”;
- Major disruptions of distribution and sales channels as consumers stayed home, and practitioners shifted patient visits to telemedicine platforms;
- Cancellation of trade shows, conferences, in-office visits, and other vital marketing and education activities.
These changes play out against extreme economic uncertainty as the pandemic drags on, and millions face unemployment and financial hardship.
Are Supplements “Essential”?
Early in the epidemic, a fundamental existential question hung over the industry: Would the dietary supplements industry be considered part of the “critical infrastructure” in a crisis like this? In other words, is it an “essential” business?
The official answer was a solid, “Yes!”
Thanks in part to the efforts of organizations like the Natural Products Association which, in March issued a letter to governors of all 50 states, nearly all stay-at-home orders included supplement companies, ingredient suppliers, health food stores and other health-related enterprises under the “essential business” umbrella.

“Designation as a critical infrastructure industry with essential products was huge. That one decision turned the fate of the industry,” Loren Israelsen, who heads the United Natural Products Alliance, one of the industry’s major trade organizations.
Rapid Responses
Relieved of the threat of total shutdown, supplement makers were able to redouble their quality assurance and employee protection efforts and focus on meeting the logistical challenges of a new and chaotic marketplace.
“I think overall we’ve done very well,” Israelsen said of the industry’s adaptation to the new post-COVID reality.
He told Holistic Primary Care that ethical, well-reputed companies already had strict environmental control systems and personal protective equipment requirements in place as part of their standard operating procedures. They were able to respond rapidly to the new situation.
“In GMP (Good Manufacturing Practices)-compliant facilities masks and eyewear are routine. The companies that did the work and the training for compliance with the Food Safety Modernization Act, were able to immediately go to their preventive control plans. They had a good understanding of where their hazard control points were.”
Many have instituted daily employee temperature checks, health screenings, and infection tracing systems. They also mandated work-from-home rules for all jobs not requiring physical presence at manufacturing and packaging facilities.
There have been a few coronavirus cases among supplement company employees. There are no reports of major multi-person outbreaks within the industry, says Israelsen, one of the architects of the Dietary Supplements Health and Education Act of 1994, which set the regulatory framework for supplements.
In terms of COVID caseloads, the supplements industry fared far better than other industries.
“Our worker safety record, to date, has been very good. Dietary supplement GMPs together with FSMA compliance have demonstrated that product quality and worker safety can and should be achievable, day in and day out.”
Israelsen added that, “the severe outbreaks of COVID in a number of meatpacking plants confirms that proper controls and a people-first system are essential for protecting workers. This means making costly changes to systems and schedules and training. I believe this ethos is embedded in our companies and so far, reports of workplace-caused infections are very low in our industry sector.”
Shortages & Slowdowns
According to Michael McGuffin, executive director of the American Herbal Products Association, botanical supplement producers have been contending admirably with significant ingredient shortages.
As the pandemic interrupted international trade, many key herbs like Turmeric/Curcumin, Boswellia, Hawthorne, Ginger, Elderberry, and Gentian were suddenly in short supply. There have also been shortfalls and shipping delays for glass bottles and grain alcohol. The latter is essential for making tinctures.
“So much of the alcohol supply is going to making hand sanitizer,” McGuffin says.
Some companies and contract manufacturers had to slow down production owing to the new employee safety requirements. “Where you’re used to having elbow-to-elbow filling lines, and now the workers are 6 feet apart, the result is lower production per shift,” McGuffin explained, adding that many companies have added more shifts to meet the soaring demand.
Rising Demand, Intensified Scrutiny
From the earliest days, it was clear that consumers considered supplements essential during the pandemic.
Industry watchers say overall supplement sales rose by more than 50% last Spring, compared with the same period in 2019.
A survey of 1,000 US consumers by Lonza, one of the world’s largest nutraceutical ingredient suppliers, showed that 44% have increased their supplement use since COVID, and 16% were stockpiling products. A consumer study by Nutrition Business Journal showed that 20% of post-COVID supplement buyers had never used supplements before.
The retail surge was matched by an equally robust growth of sales via practitioners. Kyle Bliffert, President and CEO of Atrium Innovations, a division of Nestle Health Sciences, says Atrium’s practitioner brands (which include Pure Encapsulations and Douglas Laboratories) saw sales of 120% to 150% over predicted levels in the Spring. “In March alone, total business increased by 80%.”
Typically, monthly sales are within 3% of the company’s projections, says Bliffert.
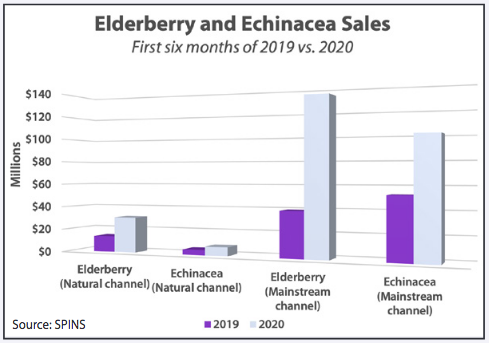
Not surprisingly, immune support products drove the surge: vitamin C, vitamin D, zinc, omega-3 fatty acids, N-acetyl cysteine, and immunomodulatory and anti-inflammatory herbs like echinacea, elderberry, and turmeric have been huge sellers.
These growth trends are not lost on the Food and Drug Administration and the Federal Trade Commission. Both have made it clear they will not tolerate COVID prevention or treatment claims for supplements, or for that matter, for off-label use of drugs. They’ve taken swift and widespread action to enforce this position.
The agencies do not consider anecdotal evidence, in vitro or animal experiments, epidemiological correlations, or even human clinical studies of other viral pathogens, to be adequate evidence to support supplement claims in the COVID context.
This puts practitioners in a challenging position: patients often turn to clinicians for intelligent, informed guidance on how to reduce COVID risk, increase immune system strength, and improve overall health. Yet federal regulators want to squelch the notion that supplements might mitigate risk. They do not want practitioners publicly promoting such ideas—at least online.
A Golden Opportunity
Despite this obstacle, the materials shortages, and the other challenges, industry leaders see COVID as a rare opportunity for the natural healthcare movement and the supplement industry to move from healthcare’s margins to the main stage.
Matt Schueller, Chief Innovations Officer for Nature’s Way, which owns the Integrative Therapeutics practitioner brand says the sweet spot for supplements is not so much in preventing or treating COVID directly, but rather “in regard to the underlying conditions and chronic diseases that predispose to COVID and raise risk of death. Integrative practitioners play a role in this area, working on prevention and root cause resolution, with a track record of safety.”
Unlike vaccines and new antiviral drugs that will take months or years to develop, integrative and functional medicine already has a wide array of readily available tools to reduce systemic inflammation, improve glucose metabolism, and improve resilience and wellbeing, all of which indirectly mitigate COVID risk.
Loren Israelsen believes the pandemic offers the supplements industry, “a unique opportunity to redefine our role in personal and public health.” It is, he says, “the single best chance we’ve ever had to establish an expanded role in the delivery of health and wellness for generations.”
A big thank-you to our sponsors: LONZA and Quicksilver Scientific. This report is only possible because of their generous support of HPC’s 2020 annual Quality Counts special report.
END







