Research on the Gut-Lung Axis, which encompasses the complex interrelationship between the gut microbiome, the immune system, and the respiratory tract, has opened a portal to entirely new ways of caring for patients with asthma and other respiratory disorders.
Supplementation with probiotics that shift microbiome production of key short-chain fatty acids (SCFAs) is showing promise for reducing lung inflammation and improving lung function in people with asthma, according to a small but important early-stage human study published in June of this year.
The study, by Nancy Wenger and colleagues at the University of Alabama, provides proof of concept for the notion that modulation of the gut microbiome can safely and positively alter the GLA, and affect lung function in patients with asthma. The findings have implications for other respiratory conditions as well.
Inter-Kingdom Crosstalk
Descriptions of the Gut-Lung Axis (GLA) began appearing in the scientific literature roughly 10 years ago, as researchers discovered that the gut microbiome exerts direct and indirect influences far beyond the GI tract, that the respiratory tract contains a microbiome, and that the lung and gut microbial ecosystems are interconnected.
In their landmark 2020 review of the subject, Raphael Enaud and his group at the Centre Hospitalier Universitaire—Bordeaux, described the GLA as a nexus of “inter-kingdom crosstalk” that plays a crucial role in maintaining healthy homeostasis.
When disrupted or dysfunctional, however, the GLA plays a part in the etiology of numerous disorders.
“The recently emerged GLA concept involves host–microbe as well as microbe–microbe interactions, based both on localized and long-reaching effects. GLA can shape immune responses and interfere with the course of respiratory diseases,” wrote Enaud and colleagues (Enaud R, et al. Front Cell Infect Microbiol. 2020).
In biomass terms, the microbiome of the lungs is far smaller than that of the gut. But contrary to a commonly held belief, healthy lungs are far from sterile: they contain roughly 10-100 bacterial cells for every 1,000 human cells. That translates into approximately 103 to 105 bacteria per gram of lung tissue, compared to 1011 to 1012 bacteria per gram in the GI tract.
In healthy people, the composition of the lung microbiome is similar to that of the healthy gut. The predominant groups (in descending order) are Firmicutes, Bacterioidetes, Proteobacteria, and Actinobacteria. Bacterial colonization of the respiratory tract begins very early in life, driven largely by translocation of microbes from the GI tract via the oropharynx.
But as with the gut microbiome, the composition of the lung ecosystem shows considerable individual variation, depending on immune system factors, environmental conditions, and presence or absence of illness and infection.
While the two ecosystems have a bidirectional relationship, the gut microbiome is clearly the dominant player. It affects lung microbial composition directly via translocation of bugs, and indirectly via microbiome metabolites that have cell-signaling and immunoregulatory effects.
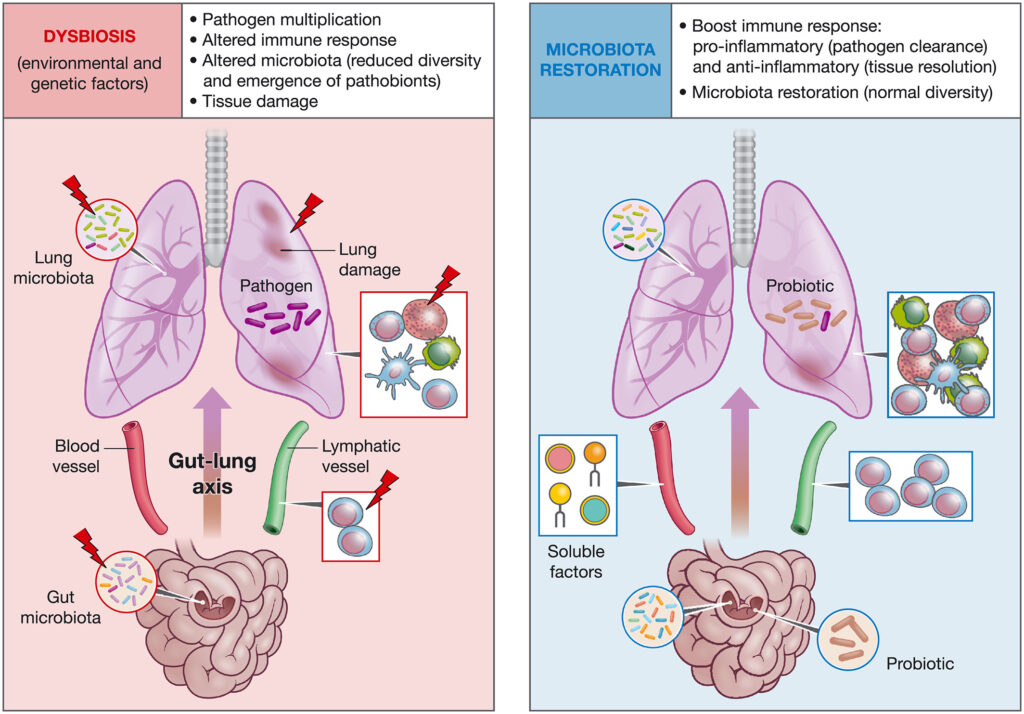
Both microbiomes contain fungi and viruses in addition to bacteria, though researchers are only just beginning to map these subdomains and characterize their complex interactions.
GLA Dysregulation
Early in the effort to describe the GLA, researchers noted correlations between certain respiratory diseases and changes in the composition of the lung microbiome. In their 2012 paper, a team at Dartmouth observed that newborns with cystic fibrosis (CF) showed lung colonization with Roseburia, Dorea, Coprococcus, Blautia, and Escherichia prior to the onset of actual respiratory symptoms (Madan JC, et al. mBio. 2012).
They saw “a high degree of concordance” between increasing and decreasing bacterial subgroups in both the gut and the lungs of the seven CF infants they tracked, noting that, “a significant proportion (14/16 genera) increasing in the gut were also increasing in the respiratory tract. For 7 genera, gut colonization presages their appearance in the respiratory tract.”
Moreover, they found that changes in the babies’ diets could cause shifts in the respiratory microflora, “suggesting a link between nutrition and development of microbial communities in the respiratory tract.” This led them to surmise that “targeted dietary or probiotic strategies may be an effective means to change the course of the colonization of the CF lung and thereby improve patient outcomes.”
Perturbations in the gut and lung microbiota caused by antibiotic drugs, poor diet, environmental and lifestyle risk factors, can disrupt mucosal immunologic tolerance early in life, predisposing people to allergies and asthma.
A number of observational studies and animal experiments have suggested that gut microbes play a role in the pathogenesis of sepsis associated with acute respiratory distress syndrome (ARDS). University of Michigan researchers backed that hypothesis by showing that bronchoalveolar lavage fluid from humans with ARDS contained an abundance of gut-specific Bacteroides species that were undetectable by culture, but that correlated with the intensity of systemic inflammation (Dickson RP, et al. Nat Microbiol. 2016)
Further, alveolar levels of TNF-α correlated strongly with these alterations in the lung microbial ecosystem. “Our results demonstrate that the lung microbiome is enriched with gut-associated bacteria in sepsis and ARDS, potentially representing a shared mechanism of pathogenesis in these common and lethal diseases.”
The lung microbiome can also influence the gut microbial environment. Rodent experiments show that influenza infection results in a rise of Enterobacteriaceae in the gut, along with decreases in Lactobacilli and Lactococci.
The GLA in Asthma
In 2010, Markus Hilty and colleagues at the UK’s National Heart & Lung Institute, used rRNA sequencing techniques to study the lung microbial compositions of 11 people with asthma, 5 with chronic obstructive pulmonary disease (COPD), and 8 healthy control subjects.
They found that pathogenic proteobacteria, particularly those of the genus Haemophilus, were much more common in the bronchi of the asthmatic and COPD subjects. On the other hand, Prevotella species were more common in the lungs of the control subjects (Hilty M, et al. PLoS One. 2010).
This is not to say that microbiome changes “cause” asthma or that asthma should be considered an infectious disease. Yet, the UK investigators posit that the presence of potentially pathogenic bugs like Haemophilus, Moraxella, and Neisseria “may have some influence on chronic airway inflammation, even when below the threshold for routine culture.”
Hilty and colleagues also point out that the marked reduction in Bacteroidetes in the lungs of people with asthma and COPD follows a similar pattern seen in the gut microbiomes of patients with Inflammatory Bowel Disease (IBD), who typically show a 10-fold lower abundance of Bacteroidetes compared to non-IBD controls (Frank DN, et al. PNAS. 2007).
Generally, the gut microbiome of people with asthma is characterized by reduced overall microbial diversity, but increased abundance of certain types of bacteria. These changes correlate with dysregulation of the immune system, which is a key feature of asthma and other chronic lung conditions.
Establishment of both the gut and lung microbiomes begins very early in life. The process can be affected by many variables including mode of birth (cesarean versus vaginal), breastfeeding, early childhood diet, food sensitivities, and early-life exposure to antibiotics. These factors, consequently, influence a child’s future risk of asthma.
Multiple Mechanisms
The gut microbiome can affect the lungs through multiple mechanisms, namely:
Immune System Modulation: Given the important role of gut bugs in educating and regulating the immune system, microbiome disruptions (dysbiosis) can cause immune dysfunction, including overactive or misdirected immune responses. Asthma may be one result of that process.
Microbial Metabolites: Gut microbes produce a host of bioactive metabolites that influence a wide spectrum of human physiology, including immune function, sleep cycles, appetite, and cognitive function. Microbe-derived short-chain fatty acids (SCFAs) like propionic, isovaleric, acetic, and butyric acids, have immunomodulatory effects. Consequently, bugs that produce these SCFAs affect the balance between pro- and anti-inflammatory functions.
Allergic Sensitization: Allergic sensitization and asthma often go hand in hand. Changes in the gut microflora have been linked to the development of allergies, suggesting another mechanism by which the microbiome can influence the development of asthma.
In short, advocates of the “Microflora Hypothesis”—as University of Michigan researcher Gary Huffnagle termed it—hold that perturbations in the gut and lung microbiota caused by antibiotic drugs, poor diet, environmental and lifestyle risk factors, can disrupt mucosal immunologic tolerance early in life, predisposing people to allergies and asthma.
Trevor Hansen and colleagues at the National Heart & Lung Institute in London, stated in a 2013 review paper that, “An understanding of the role of microbes in the atopic march towards asthma, and in causing exacerbations of established asthma, provides the rationale for new specific treatments that can be assessed in clinical trials.”
New Therapeutic Possibilities
These new therapeutic possibilities include use of antibiotic drugs, probiotics, prebiotics, and fecal microbiota transplantation. Research on all these options is still in very early stages, but the door is wide open.
In his prescient 2010 review, Huffnagle states that “The composition of the microbiota can be manipulated by combinations of antibiotics, probiotics, and dietary components.” These treatments, used in combination with other dietary factors such as fatty acids and phenolic compounds, could potentially “have direct growth promoting or inhibiting activity for specific microbes.” That, in turn, could play a role in preventing and treating conditions like asthma.
C. Vivek Lal, MD, a pediatric intensive care physician at the University of Alabama, Birmingham, wants to make good on the therapeutic promise described by Huffnagle and others more than a decade ago.

In addition to his ICU work, Lal is Director of Clinical Innovation at UAB’s Heersink Institute for Biomedical Innovation. He has been researching the mechanisms of microbiome signaling they pertain to lung function for more than a decade, and translating the findings into innovative products in both the dietary supplement and prescription drug markets.
“The microbiome field is very interesting because changes in the microbiome create foundational systemic effects,” Lal told Holistic Primary Care in a recent interview. Via a company called Resbiotic, Lal and colleagues have developed and brought to market a unique formulation of three patented probiotic strains combined with medicinal herbs thought to improve lung function.
The product, called ResB Lung Support, contains:
- Lactiplantibacillus plantarum RSB11™(8.25 × 109 CFU), Lactobacillus acidophilus RSB12™(7.9×109 CFU), and Lacticaseibacillus rhamnosus RSB13™(6.4 × 109 CFU)
- Vasaka (48 mg per capsule): Also known as Adhatoda vasica or malabar nut, Vasaka is an Ayurvedic herb with a long history of use as a respiratory remedy. The leaves contain two key alkaloids–vasicine and vasicinone—that may contribute to the plant’s expectorant and bronchodilatory effects.
- Turmeric (42 mg): Now one of the world’s most popular botanical medicines, turmeric (Curcuma longa) has immunoregulatory and antioxidant properties.
- Tulsi (42 mg): Another ayurvedic mainstay that has become popular worldwide, Tulsi (Ocimum tenuiflorum)—also known as Holy Basil—supports immune system balance. It is also a good antioxidant.

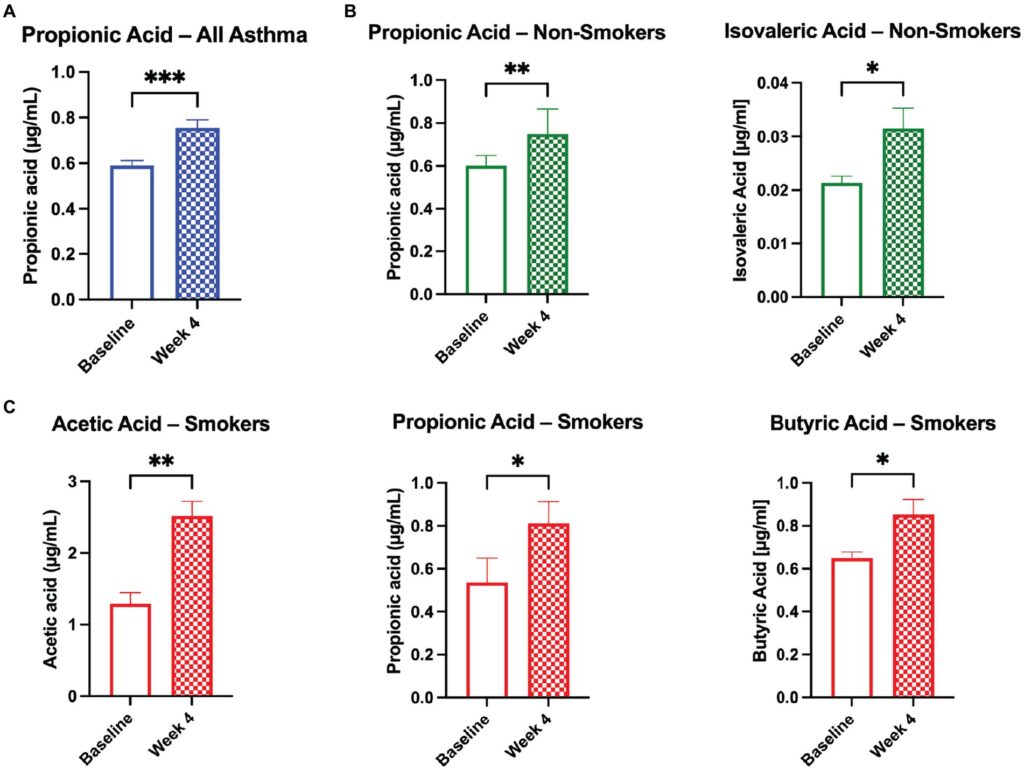
As shown in a recent clinical trial sponsored by Resbiotic and conducted at the University of Cork, Ireland, 11 subjects with asthma who took ResB twice daily for 4 weeks, showed marked increases in circulating levels of propionic, isovaleric, acetic, and butyric acids (Wenger NM, et al. Front Nutr. 2023). These particular SCFAs affect immune cell function, and also modulate a variety of inflammatory pathways, including TNF-α, IL-2, IL-6, and IL-10.
Notably, they did not find similar SCFA increases in the 11 healthy, non-asthmatic control subjects who also took the ResB supplement.
The authors hypothesize that the gut dysbiosis common in asthmatic individuals diminishes the normal metabolism of anti-inflammatory SCFAs, impairing the body’s ability to regulate systemic inflammation, and exacerbates allergic lung inflammation.
Improved Lung Function
This was an open-label safety study, not a formal treatment trial. But the investigators did gather data on lung function as measured by spirometry (FEV1 and FVC); changes in oxygen levels (% pulse oxygen levels), and participant scores on the St. George’s Respiratory Questionnaire (SGRQ) at baseline and after 4 weeks.
They found that among the asthmatic subjects, average FEV1 increased significantly (p = 0.018) and FVC trended upward (p = 0.082), though the latter change was not statistically significant. The non-asthmatic control group showed no such changes.
For the cohort as a whole, there were no major changes in total SGRQ scores from baseline to completion of treatment. However, in the asthmatic subjects and those who smoke, 36% answered that they had improvements in overall health, 43% coughed less frequently, 43% experienced fewer instances of shortness of breath, and 29% reported fewer instances of breathing-related sleep disturbances.

There were no serious adverse effects associated with ResB supplementation, and none of the subjects stopped treatment for any reason. One experienced bloating, though this was not problematic. Ninety percent of asthmatic participants and 82% of healthy ones said they would recommend the treatment to family members and friends.
The ResB formula, Dr. Lal explained, works via multiple mechanisms: the herbs downregulate inflammation, quench free radicals, and promote bronchodilation and mucus clearance, while the probiotic organisms normalize the gut microbiome and increase production of anti-inflammatory SCFAs which tend to be deficient in people with asthma.
This is not the only recent study to suggest that Lactobacillus probiotics have a role in ameliorating asthma.
Sina Sadrifar and colleagues at the Semnan University of Medical Sciences in Iran treated 40 people with mild to moderate asthma using Lactocare, a 7-strain probiotic product containing: Lactobacillus casei (3 × 109 CFU/g), L. acidophilus (3 × 109 CFU/g), L. rhamnosus (7 × 109 CFU/g), L. bulgaricus (5 × 108 CFU/g), Bifidobacterium breve (2 × 1010 CFU/g), B. longum (1 × 109 CFU/g), and Streptococcus thermophilus (3 × 108 CFU/g), plus 38.5 mg fructo-oligosaccharide as a prebiotic.
Study participants were randomized to Lactocare, once daily after lunch, or a starch placebo for 8 weeks.
Spirometric measurements showed significant increases in FEV1 and FVC in the people taking the probiotic supplements. The investigators saw no such change in the placebo group. The improvements in lung function correlated with reductions in serum levels of Th2-cell associated IL-4, suggesting an indirect anti-inflammatory effect resulting from changes in the microbiome (Sadrifar S, et al. Allergy Asthma Clin Immunol. 2023).
Research into the role of the GLA in lung disease is still in an early stage, but already it is yielding new ways of treating people with asthma and other chronic conditions.
Dr. Lal told Holistic Primary Care that several other studies of ResB are now in the works, including a 3-month multicenter RCT looking at the impact of the product in patients with COPD and non-CF bronchiectasis, and another exploring its potential in the context of recovery from viral infections, particularly pneumonia in elderly people.
“We are also looking at its impact on neutrophilic inflammation, which will have implications for long Covid,” says Lal.
END







