The typical human body contains roughly 1.5 kilograms of microorganisms. That’s about equal in weight to the average human brain.
It’s an interesting equivalence, given what we are learning about the myriad ways in which the gut microbiome influences the brain and central nervous system.
Many common mental illnesses, including severe depression and anxiety, are directly and indirectly connected with distinct microbiome changes. Alterations in the gut microbiome may also play a role in autism and Parkinson’s disease.
The Gut-Brain-Microbiome Axis—the aggregate term for the web of relationship between the microbiome, the digestive organs, and the nervous system–functions in bi-directional manner. The microbiome affects the nervous system, and vice versa.
This has wide-ranging and exciting implications for healthcare. Emerging evidence points to encouraging new treatments for mental, cognitive, and behavioral disorders.
A Mental Health Revolution
Ted Dinan, MD, PhD, is one of the world’s foremost researchers in this rapidly expanding field. A psychiatrist by training, Dr. Dinan is principal investigator at APC (Alimentary Pharmabiotic Center) at University College, Cork, an interdisciplinary institute entirely focused on nutrition, the microbiome, and how they affect human physiology.
Dr. Dinan has studied the gut-brain-microbiota axis for more than thirty years. He believes “psychobiotics” are poised to revolutionize mental healthcare.

A psychobiotic is defined as, “a live organism—usually a bacterium—which when ingested in adequate amounts, produces a positive mental health benefit in patients suffering from a psychiatric illness.”
In a recent webinar sponsored by Howaru probiotics and Holistic Primary Care, Dr. Dinan summarized the current understanding of the brain-gut-microbiota axis and the potential role of psychobiotics in management of common psychosocial, cognitive, and neurological conditions.
He stressed that not all probiotics are psychobiotics. But several strains of Lactobacilli and Bifidobacteria used in probiotic products are “capable of producing and delivering neuroactive substances like GABA and serotonin which act on the gut-brain axis.”
In fact, all five major human neurotransmitters—GABA, Serotonin, Norepinephrine, Dopamine, and Acetylcholine—are produced at least to some degree by commensal gut microbes (Wall R, et al. Adv Exp Med & Biol. 2014).
Dinan’s team has looked in depth at various species of Lactobacilli. They’ve found that all strains produce GABA. Some produce more and others less, but all are capable of synthesizing this key inhibitory neurotransmitter.
Most microbiome-derived neurotransmitters act locally on the enteric nervous system (ENS) rather than in the brain itself. But the ENS also interfaces with the autonomic nervous system through the vagus nerve—exerting an indirect influence on the brain.
Sizing Up Strains
Last year, Lotte Stenman and colleagues at DuPont Nutrition & Biosciences in Finland profiled 12 strains of Lactobacilli and Bifidobacteria in animal models, looking for signs that these bugs might impact cognition and behavior in ways relevant to humans. They used four well-validated rating scales to assess stressed and unstressed mice.
Of the 12 strains, three showed possible benefits for human mental health: Lactobacillus paracasei Lpc 37, L. plantarum LP12418, and L. plantarum LP12407 (Stenman L, et al. Behav Brain Res. 2020). All three reduced stress-related behaviors and deficits of learning and memory in the stressed animals, and mitigated behaviors indicative of anxiety and depression.
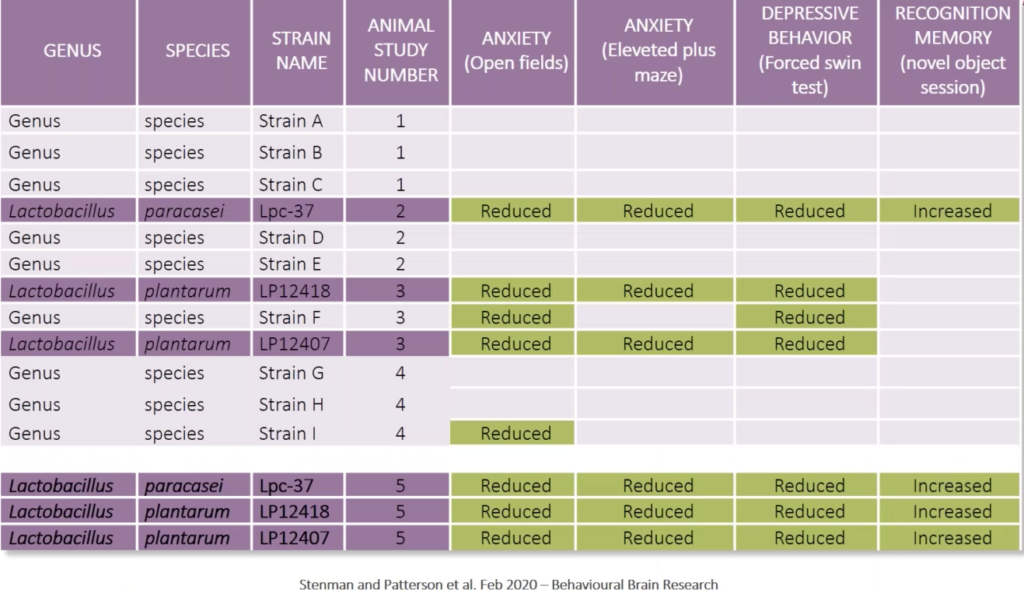
Dr. Dinan noted that over the last year, there’s been translational work with these strains to elucidate mechanisms of action.
The Lpc 37 strain dramatically reduces corticosterone—the rodent equivalent of cortisol—in the stressed mice. This reduces stress behavior.
LPC 12418 significantly increases GABA neurotransmission. “GABA receptors are where benzodiazepines act. So it makes sense that this could mitigate anxiety,” says Dinan.
Some strains of Bifidobacterium longum also show potential. Dinan’s group studied 22 healthy humans and found that compared with an inert placebo, supplementation with a strain called B. longum 1714 reduced morning salivary cortisol. Participants taking the active probiotic reported less subjective stress, and these changes correlated with EEG evidence of stress mitigation (Allen AP, et al. Transl Psychiatry. 2016).
“This was the first study to demonstrate EEG changes in the subjects taking the psychobiotic. We were looking at a number of electrophysiological measures. We looked at frontal mobility, which is often altered in anxiety, and found this was radically changed when the subjects ingested this particular psychobiotic. It seemed to have antianxiety or anxiolytic effects.”
The APC group then studied the same B. longum strain in university students over two semesters. In the first semester, before exams, they took either a placebo or the B. longum probiotic. In 2nd semester they switched treatments.
“What we found was that the Bif longum seemed to increase sleep duration, relative to that seen with the placebo. A number of studies have emerged in recent months suggesting that psychobiotics can positively modulate sleep.”
An Emerging Field
Research on psychobiotics is still in its infancy, but already there are human studies showing that targeted probiotics can improve both subjective and objective measures of mental and neurological illness.
That’s very good news, because we clearly need better approaches for the management of mental illnesses.
According to the National Institute of Mental Health, tens of millions of Americans suffer from some form of mental illness but only about half are being treated. Modalities like psychotherapy are inaccessible to many, owing to geographic, economic, or cultural barriers. While the medications that we prescribe can be effective for some, they have clear shortcomings and side effects.
Clinically, we’ve known for some time that mental states can have profound implications for the GI system. Most patients with functional pathologies like Irritable Bowel Syndrome know that stress and anxiety can trigger and exacerbate symptoms. We are finding out that the composition of the gut microbiome impacts the nervous system, affecting how we think and feel.
Reciprocal Maintenance
The average person hosts more than 1,000 species of microorganisms; most are long-term residents in the GI tract. Each of us carries about one hundred times more microbial DNA than human DNA. These organisms produce essential biochemical building blocks.
“It’s just as well that we’ve co-evolved with microbes,” said Dr. Dinan. “We feed them, and in turn they produce the molecules that our brains and other organs need.” He believes the microbiome is actually a reservoir of additional DNA and genetic machinery that our “human” cells could not maintain on their own.
“If you were to take the DNA from the bacteria whose output we require, our cells would actually not be big enough to hold all of it.”
Essential for Neurogenesis
The brain and central nervous system cannot develop normally without the presence of gut bacteria.
The University College Cork, where Dinan is based, has a sterile lab in which researchers can raise animals without any gut microbiota. Suffice to say, these animals are quite abnormal.
“Myelenation, which is so important for signal transmission in neurons, is not normal in the prefrontal cortex if an animal does not have a gut microbiome.”
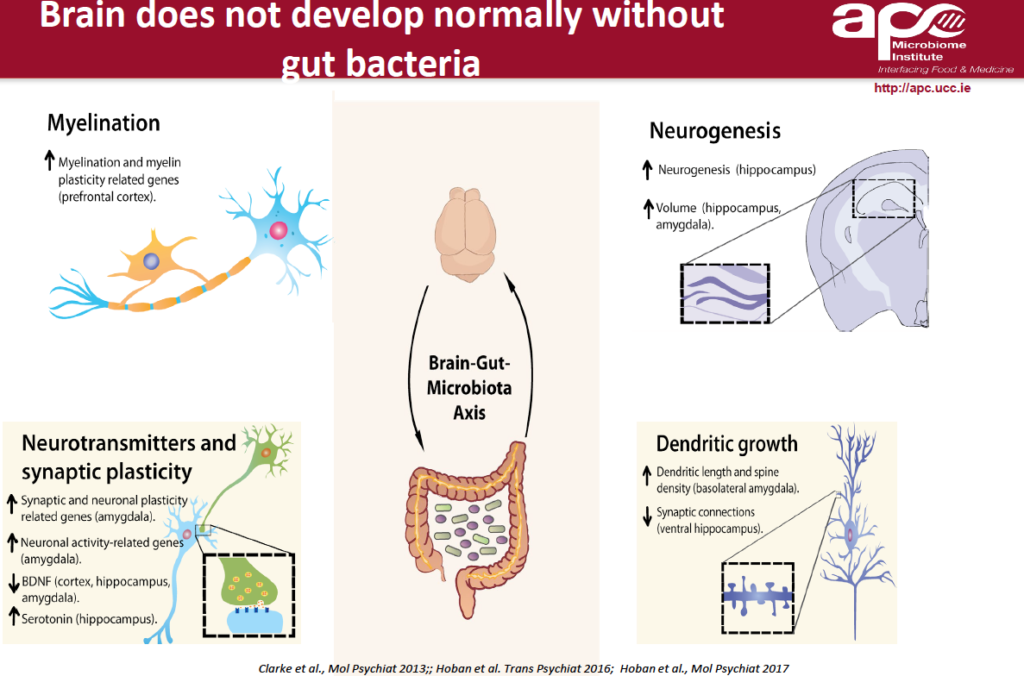
In certain parts of the brain, such as the hippocampus, there are stem cells or progenitor cells that differentiate to form neurons. “We know that this form of neurogenesis is altered in germ-free animals. Also, the connections—the dendritic connections between neurons—is also altered. And the behavior in these germ-free animals is highly abnormal.”
Mice, like humans, are social creatures. Given the opportunity to interact with an inanimate object or another mouse, an ordinary healthy mouse will invariably choose the other mouse. Microbiome-free mice behave differently. “If you give them the opportunity to interact with another mouse or, say, a pen, they are as likely to interact with the inanimate object as they are to interact with the other animal.”
What Happens in Vagus…
From a clinical perspective, three components of the gut-microbiome-brain axis play particularly significant roles: the vagus nerve; bacterially-derived short chain fatty acids (SCFAs); and microbial production of tryptophan and other metabolites.
The vagus is the unifying link in the axis. It governs parasympathetic communication, and has a particularly important role in gastrointestinal signaling and control—the so-called “rest and digest” processes.
In experiments with mice, Dinan has found that introduction of certain gut bacteria can provoke behavior indicative of anxiety. But the same bacteria had no effect on mice in which the vagus nerve was severed.
“Our group in Cork were the first to show that certain microbes could only communicate with the brain if the vagus nerve was intact. If the vagus is cut, the microbes cannot communicate with the brain.”
A Scandinavian study of people who’d had vagotomies led to the hypothesis that microbiome changes might play a role in the etiology of Parkinson’s disease.
Vagotomy was widely used to treat severe peptic ulcers back in the 1960s and ‘70s. People who’d had the procedure then had far lower incidence of Parkinson’s as they aged, compared to age-matched people with intact vagus nerves.
It turns out that alpha-synuclein, a protein that damages dopaminergic neurons and that plays a role in Parkinson’s, originates in the gut, not in the brain itself, Dinan explained.
More recently, a small study but compelling new study showed that fecal transplant from healthy subjects to Parkinson’s patients reduced many of the psychological as well as motor dysregulations associated with the disease (Xue LJ, et al. Medicine. 2020).
“This raises all sorts of interesting possibilities,” says Dr. Dinan.
Microbial Metabolites
Gut microbes produce a host of metabolites, including various short chain fatty acids and lipopolysaccharides. Some of these bioactive metabolites can be absorbed into the bloodstream just like other digested nutrients. Once absorbed, they exert effects on remote sites like the brain.
Microbially-derived SCFAs, produced when the bugs digest dietary fiber, are important modulators of gut-brain communication. SCFAs like butyrate, acetate and propionate are particularly important because they are epigenetic modulators.
SCFAs can travel to the brain, where they interact with G-protein coupled receptors. But their biggest effect may be as regulators of gene activity. Butyrate, in particular, is an inhibitor of histone deacetylase (HDAC); it can change gene expression in the brain and other organs.
Microbiome-derived metabolites also indirectly influence the CNS by acting locally on enteric endocrine, immune, and nerve cells.
Microbial metabolites also influence enzymes crucial for neurotransmitter production. Some up-regulate enzymes that turn tryptophan into serotonin, while down-regulating pathways that use tryptophan to make kynurenine—a molecule that signals distress.
Though it is a fundamental building block for serotonin, tryptophan is stored in very small quantities in the brain. We get some from our diets, but much of it is actually produced by Bifidobacteria and other microbes in the GI tract.
Transferring the Blues
Many human mental illnesses have distinct biochemical ‘signatures’ that influence and are influenced by the gut microbiome.
Dinan’s team studied the microbiome composition of severely depressed patients versus age- and sex-matched control subjects. The depressed people had considerably less diverse, and less rich microbiota. There were no dietary factors that could account for the observed differences.
What’s really interesting is that the Cork team was able to, essentially, transfer the depression phenotype from human subjects into rats via fecal transplantation.
“We did a study transplanting microbes from healthy versus depressed humans into rats. In the rats that got microbiome fecal transplants from healthy subjects, the physiology was healthy, they behaved normally, showed normal biochemistry, and the immunology was normal. When the rats got microbiota from depressed humans, they developed many of the features we would associate with human depression–anxiety, anhedonia, inflammation.” (Kelly et al. J Psychiatr Res, 2016)
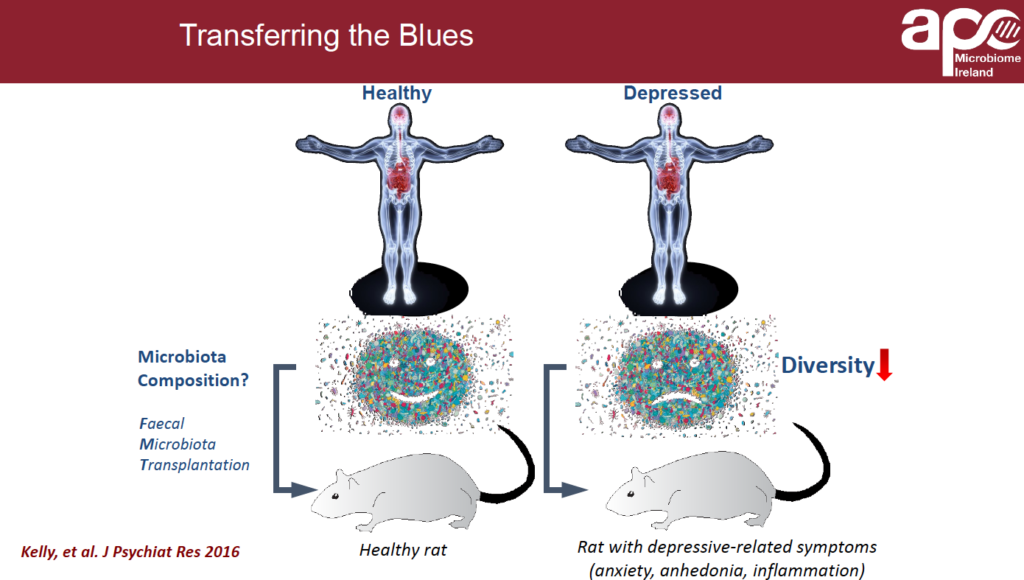
In a very real sense, they “transferred the blues” from depressed humans to normal non-depressed rodents.
These were not just subjective impressions. There were measurable changes in tryptophan metabolism and in biochemical indicators of a pro-inflammatory phenotype.
Measurable Brain Changes
Researchers worldwide are now studying interactions between the microbiome and the nervous system.
In 2018, Deepika Baga and colleagues at the University of Graz, Austria, published two elegant studies using functional MRI imaging to document how microbiome changes affect the human brain.
Both papers were based on data from forty-five healthy subjects, aged 20-40 years, subdivided into three equal groups: one got a multi-strain probiotic (Ecologicâ825, manufactured by Winclove Probiotics) for four weeks; the second got a placebo identical in appearance to the probiotic; the third got no intervention.
The probiotic, available commercially as OmniBioticâ, is a freeze-dried powder; each 3-gram sachet contains 6 species of Lactobacillus, and 3 species of Bifidobacterium. Participants took one sachet per day for 4 weeks. They underwent fMRI at baseline, and again after the 4-week period.
Dr. Bagga’s team used several well-validated rating scales (Positive and Negative Affect Schedule, Symptoms Checklist-90, Allgemeine Depressionsskala, Leiden Index of Depression Severity) to measure affect. They tracked fMRI changes while the subjects did emotional decision-making and emotional recognition memory tasks.
There were major differences between the probiotic-treated people and the controls.
The probiotics improved the behavioral scores on the depression and anxiety questionnaires, “significantly increasing positive affect and blunting vulnerability to depression in terms of hopelessness (HOP) and risk aversion,” the authors note (Bagga D, et al. Gut Microbes, 2018.
“The marked beneficial effects observed in the hopelessness and risk aversion subscales…suggest an influence of probiotic intake on cognitive mechanisms associated with vulnerability to mood disorders. On a similar note, increase in PANAS positive affect indicates improvement in general wellbeing.”
These findings corroborate previous studies showing that manipulation of gut microbiota influences mood and cognition.
Increased Connectivity
The fMRIs showed that daily probiotic intake led to “a significant difference in brain activity in cingulum, precuneus, inferior parietal lobule, thalamus, and parahippocampal gyrus.”
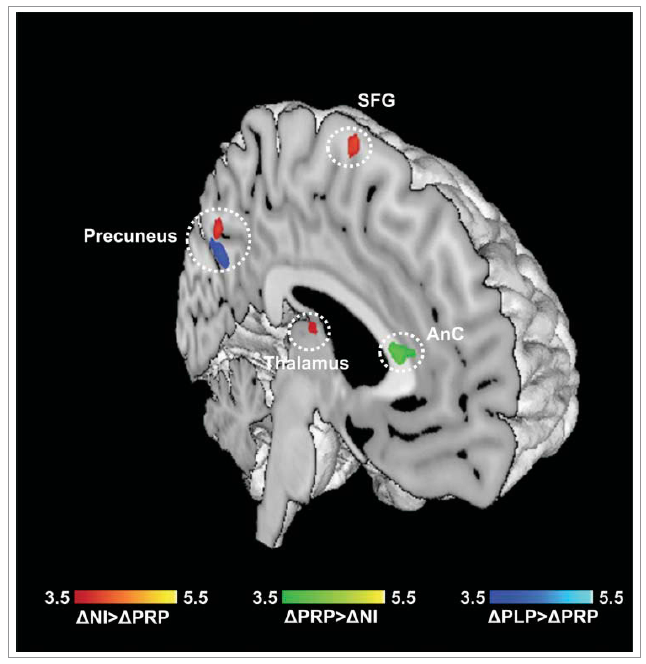
The cingulum is a point of connection between brain regions involved in cognitive control, reasoning, decision-making and problem-solving. “The anterior cingulum primarily mediates emotional processes while the middle and posterior divisions specialize in higher order cognitive processes and memory formations,” Dr. Bagga writes, adding that “the role of the precuneus in decision-making processes is well documented.”
In the second paper, Bagga’s group documented functional connectivity changes in several brain networks among the subjects taking the probiotic formula, but not in the controls—compelling evidence that the gut microflora can alter brain connectivity and function, though there were no structural changes (Bagga D, et al. Eur J Nutr. 2019)
“Functional connectivity (FC) changes were observed in the default mode network (DMN), salience network (SN), and middle and superior frontal gyrus network (MFGN) in the Probiotic group as compared to the placebo probiotic and (no-treatment) control groups.”
Real World Impact
Can we expect that physiologic and functional changes like these would translate into improved real-world clinical outcomes for patients?
A recent meta-analysis suggests that they do.
Researchers at Beijing University of Chinese Medicine published a systematic review and meta-analysis seven randomized, placebo-controlled studies investigating the role the microbiome and probiotics play in stress resiliency.
These seven trials represented an aggregate total of 1,146 subjects who took different types of probiotics. Outcomes were measured using validated rating scales like the Perceived Stress Scale and the Berocca Stress Index.
The authors concluded that probiotics can reduce subjective stress levels in heathy volunteers and may also relieve stress-related subthreshold anxiety/depression levels. The standard mean difference (SMD) was -0.14 for overall stress data in the meta-analysis with a confidence interval of -0.27 to -0.01 and a p-value of 0.03; indicating a small, but statistically significant improvement.
Stress related subthreshold anxiety/depression was also improved in the treatment group with an SMD of -0.13 (CI -0.26 to 0.00) and a p-value of 0.05 (Zhang N, et al. Brain and Behavior. 2020)
Probiotics could help women avoid post-partum depression. Slykerman and colleagues at the University of Auckland, New Zealand studied 423 women in the second trimester of pregnancy, randomized to either placebo or a Lactobacillus rhamnosus probiotic. They took their assigned treatments through the course of pregnancy and until 6 months post-partum. The women on the probiotic had far lower rates of anxiety and depression (Slykerman RF et al. EBioMedicine. 2017).
“This is a very well-powered study. It does show that this particular L. rhamnosus has the capacity to reduce postnatal depression rates relative to placebo,” said Dr. Dinan, adding that up to 20% of all women experience post-natal depression. “Anything that ameliorates this is clearly of relevance.” The findings are especially important because probiotics are safer than prescription antidepressants during pregnancy.
Microbiome & Aging
The health of the gut microbiome is affected by many things: diet, exercise, environmental exposures, presence or absence of other diseases, use of probiotics, prescription drug, and age.
The latter variable is complex. In general, as people age, their microbiomes become less diverse and less healthy. But the change may reflect the fact that older people tend to eat more limited diets, they exercise less, and they’re often on multiple drugs. It may not be an inevitable consequence of aging.
Andrea Ticinesi and colleagues at the University of Parma’s Microbiome Research Hub, have looked closely at the impact of aging on the microbiome and vice versa.
In one study, they compared microbiome composition in 25 healthy octogenarians, and 25 hospitalized people of similar age and background. Among the healthy elderly, the microbiota resembled that of younger people, while the ill hospitalized subjects showed the diminished microbial diversity thought to be typical of advanced age.
Ticinesi’s group has also shown a strong correlation between lack of microbial diversity, sarcopenia, and frailty: frail elders show less microbial diversity than robust people of the same age and ethnicity. These observations prompt the question of whether there is a “microbiome-muscle axis” (Ticinesi A, et al. Nutrients. 2017).
There is still much to learn about how to intervene in positive ways to improve the health of the gut-brain-microbiome axis (read Optimizing the Gut-Brain Axis). Though many questions remain, we’ve garnered evidence that the microbiome has measurable effects on the brain and that probiotic optimization through more exercise, better quality foods, and supplementation can improve clinical symptoms.
END







