They say the way to a person’s heart is through the stomach. The same could be said about a person’s brain.
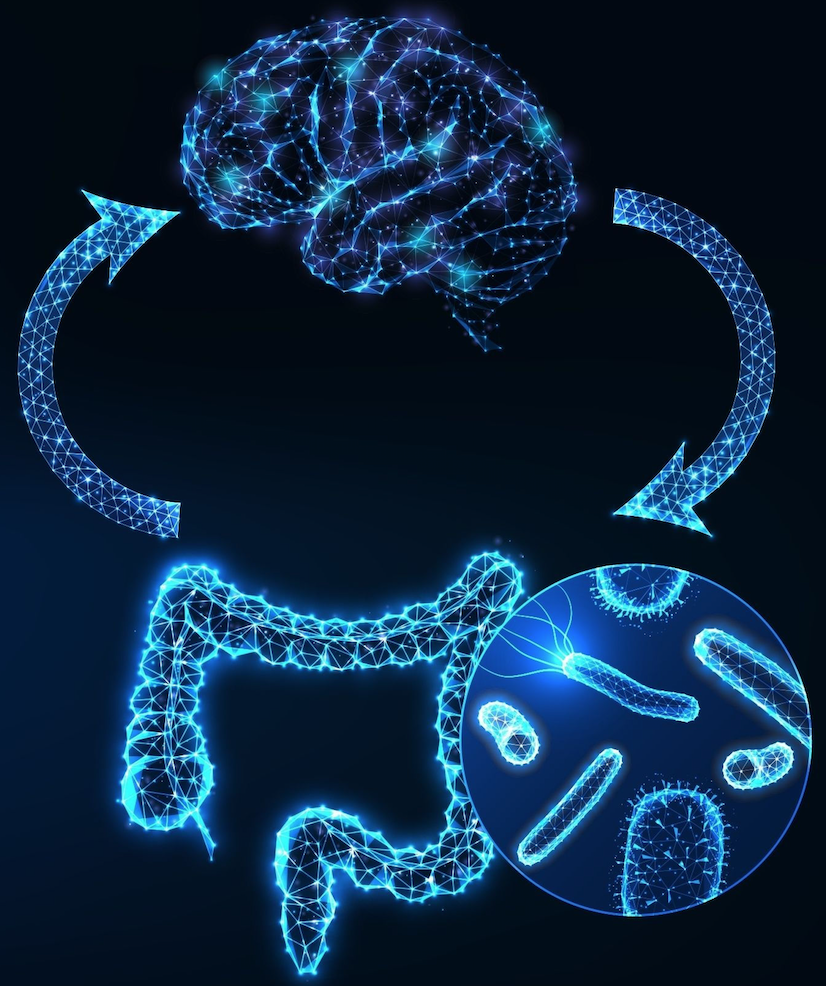
With all that we’re now learning about the gut-brain axis, it is becoming clear that mood, cognitive function, sleep cycles, and many other aspects of our psychological health are directly influenced by factors originating in the gastrointestinal tract.
Consider serotonin, the neurotransmitter that regulates mood, especially positive affect: roughly 90% of the body’s serotonin is produced in the gut, not the brain.
If the gut is not healthy, it is difficult for someone to experience optimal mental and cognitive health. GI dysfunction and poor diet predispose people to depression, anxiety, and a host of other problems.
The conventional medical model encourages practitioners to focus “above the neck” when working with patients struggling with mood disorders. But these problems are not just in people’s brains. To truly improve outcomes, we need to focus on what’s happening in the gut.
Serotonin, also known as 5-hydroxytryptamine (5-HT), is a neurotransmitter that connects the nerve cells in the brain to the entire nervous system. Beyond its role in influencing mood, it is also involved in a plethora of important bodily functions including: temperature regulation, sleep cycles, appetite, digestion and nutrient absorption, wound-healing, blood clotting, sexual function, learning, and enforcing memory.
The conventional medical model encourages practitioners to focus “above the neck” when working with patients struggling with mood disorders. But these problems are not just in people’s brains. To truly improve outcomes, we need to focus on what’s happening in the gut.
Serotonin production in the gut is greatly affected by the state of the gut microbiome, and by food sensitivities and autoimmune reactions, which can seriously disrupt GI function. Consequently, food sensitivities can play a significant role in mood disorders. Serotonin imbalances can negatively affect mental health and is associated with mood disorders, depression, anxiety and panic attacks.
Mind & Microbiome
Beyond serotonin, the gut microbiota also produces and modulates GABA, dopamine, glutamate, and many other signaling molecules. Communication between the gut and the brain is constant and bidirectional.
A new review paper by Nirav Batia and colleagues provides a thorough description of the “biological link between the microbiota, immune signaling, and the CNS suggesting that microbial metabolites could regulate both neurological and immunological activities in the brain.” (Bhatia NY, et al. CNS Neurol Disord Drug Targets. 2022).
Another recent paper by Schrodt and colleagues compiles data from 76 human and animal studies showing links between changes in the relative prevalence of different gut organisms and patterns of neuroinflammation and depression.
The authors underscore the connection between increased intestinal permeability (aka “leaky gut”) and depression, and also point out the disruptive effect of antibiotic drugs (Schrodt C, et al. Nutr Neurosci. 2022).
Leaky Gut and Depression
A new study by researchers based at the Ovidius University of Constantina, Romania, addresses the question of “whether intestinal permeability syndrome is correlated with depression” in patients with inflammatory bowel disease (IBD) or Crohn’s disease.
“Our results highlighted a correlation between depression and calprotectin and LBP, which contributes another step to the rapid identification of biomarkers and can indicate the existence of depression.”
Iordache MM, et al. J Clin Med. 2022
To find out, they measured levels of calprotectin, zonulin, lipopolysaccharide-binding protein (LBP) and other key permeability markers in a cohort of 30 patients with IBD or Crohn’s, and plotted these markers against levels of depression, as indicated by Patient Health Questionnaire (PHQ-9) scores. More than half of the patients had PHQ scores indicating depression.
There were statistically significant correlations between depression scores and circulating levels of calprotectin and LBP, both of which were elevated above the normal range in this study cohort.
Somewhat surprisingly, there was no correlation between depression scores and zonulin levels. None the less, the authors believe their data confirms the notion that increased intestinal permeability, chronic inflammation, and depression are inter-related.
“Our results highlighted a correlation between depression and calprotectin and LBP, which contributes another step to the rapid identification of biomarkers and can indicate the existence of depression.” (Iordache MM, et al. J Clin Med. 2022).
The Scrhodt study is important because it highlights the links between leaky gut—which is often caused by food sensitivities to dietary proteins—and a very common and often debilitating mood disorder.
Leaky gut can be caused by food reactivity and sensitivity. Intestinal permeability can also lead to immune dysregulation and autoimmune disorders, both of which can affect mental health of a patient. It becomes a vicious feed-forward cycle, though patients may not be aware of the connections or the triggers.
Given that serotonin affects both mood and digestive function, it is not entirely surprising that 50 to 90 percent of people with IBS are also found to have psychological conditions including anxiety or depression.
It makes good clinical sense to explore questions about diet and GI function in all patients who are struggling with depression, anxiety, or other mood disorders, even in those patients—or especially in those patients—who don’t mention dietary or digestive concerns.
IBS and Serotonin
Given that serotonin affects both mood and digestive function, it is not entirely surprising that 50 to 90 percent of people with IBS are also found to have psychological conditions including anxiety or depression.
One hypothesis to explain the connection holds that production of serotonin reuptake transporter (SERT) molecules is deficient in the enterocytes of people with IBS. According to a recent review article by Bruta and colleagues, several studies suggest that individuals with IBS—especially the constipation-dominant form of the disease—typically show specific polumporphisms in the gene coding for SERT (Bruta K, et al. Translat Med Comm. 2021).
But diet also plays a role. The authors note that a hypercaloric high-fat, high sugar diet reduces SERT binding by as much as 30%.
Identifying Common Triggers
All of this new research points to the possibility that we can support healthy brain function through dietary changes and interventions aimed at healing the gut mucosa and restoring a healthy gut microbiome. The first step is to identify the food sensitivities and triggers that cause the breakdown of the intestinal barrier.
Common foods that can increase intestinal permeability include gluten, the bovine milk protein butyrophilin (BTN) and other dietary proteins. Gluten is found in foods that contain wheat such as bread, cereal, pasta and even beer. Reactive dietary proteins can be found in a variety of other foods including corn, soybeans, spinach, tomato, and of course dairy products such as milk, cheese, yogurt, and butter.
This is just a small list which can serve as a starting point. The potential triggers of immune dysregulation are myriad, and highly variable from person to person.
Cyrex Laboratories, a clinical lab specializing in functional immunology and autoimmunity, offers several test arrays which can be valuable tools for identifying triggers and optimizing a patient’s diet.
One such test is a panel called the Array 10 – Multiple Food Immune Reactivity Screen™. It is the result of 30 years of scientific development, and evaluates the immune reactions to foods in both raw or modified (cooked) forms, as well as food enzymes, lectins, and artificial food additives such as “meat glue” (transglutaminase), colorings, and gums.
The Array 10 enables early detection of dietary triggers of immune dysregulation and autoimmune reactivity. It gives doctors and patients a preventive test, which can provide insights so that patients can modify their diets in advance of GI or mental health problems. The test array can also be used to monitor the effectiveness of customized dietary protocols.
Cyrex provides a number of other useful testing arrays: Array 2 evaluates intestinal barrier integrity, Array 3X assesses wheat and gluten antibodies as well as antibodies to gluten-related self-enzymes. The Array 4 identifies foods that are cross-reactive to gluten as well as common dairy protein immune reactions.
Knowing which foods trigger reactions and can lead to intestinal permeability will help you create tailored diet plans for your patients. Ultimately this will lead to a healthier gut, better serotonin balance, and improved physical as well as mental and emotional health.
END
Chad Larson, NMD, DC, CCN, CSCS, holds a Doctor of Naturopathic Medicine degree from Southwest College of Naturopathic Medicine, and a Doctor of Chiropractic degree from Southern California University of Health Sciences. He is a Certified Clinical Nutritionist and a Certified Strength and Conditioning Specialist. He particularly pursues advanced developments in the fields of endocrinology, orthopedics, sports medicine, and environmentally induced chronic disease. He is an advisor and consultant on the Clinical Consulting Team for Cyrex Laboratories.
Mark R. Engelman, MD, is the Founder and President of the Engelman Health Institute. He served for 23 years as the director of St. Joseph’s Medical Center emergency department. He was president of the Maricopa County American Heart Association; Chief Physician for the Arizona Boxing Commission; and Founder and CEO of AmeriMed American Hospitals in Mexico. Dr. Engelman speaks nationally and internationally on emergency medicine, as well as immunology. He is the Director of Clinical Consulting for Cyrex Laboratories.