
Use of marijuana during pregnancy, especially in the first trimester, significantly raises the risk of preterm birth, low birth weight, and perinatal complications that require neonatal intensive care.
Those are the main conclusions of a massive metanalysis and systematic review of 57 studies looking at the impact of in utero cannabis exposure. Combined, the studies included in this metanalysis represented close to 13 million mother-infant pairs; in over 100,000 of them, the women reported using cannabis while pregnant.
Researchers have known for a long time that Δ9-tetrahydrocannabinol (THC) can cross the placenta, and that the endocannabinoid receptor system plays a central role in multiple processes of fetal brain development. These facts have led many to hypothesize that intrauterine exposure to THC—and possibly, other cannabinoids—could adversely affect early childhood neurocognitive development.
“Our meta-analysis suggests that infants prenatally exposed to cannabis are more likely to be born preterm, with a low birth weight, and require NICU admission than infants without cannabis exposure.“
–Maryam Sorhkou, Centre for Addiction and Mental Health, Toronto
Over the years, there have been many studies from all over the globe attempting to identify a causal relationship. The findings from these studies have been quite variable.
Massive Effort
The current metanalysis, a collaboration between researchers at the Centre for Addiction and Mental Health, Toronto, and the Tasmania Centre for Mental Health Service Innovation, University of Tasmania, Hobart, Australia, is the most comprehensive effort to date, aimed at answering the question of how maternal cannabis use affects fetal and early childhood development.
“Information on long-term outcomes in children prenatally exposed to cannabis is sparse, with few longitudinal cohorts extending beyond infancy,” writes lead author Maryam Sorkhou, a PhD candidate at the Toronto center. “This systematic review and meta-analysis synthesized prospective and cross-sectional human studies to measure the effects of intrauterine cannabis exposure on birth, behavioral, psychological and cognitive outcomes in infancy until early childhood.”
Dr. Sorkhou and her colleagues initially identified 932 studies that were in some way relevant to the topic. Of these, 57 met the team’s rigorous eligibility criteria. The authors note that all of the studies they included had scores of between 6 and 9 on the Newcastle Ottowa Scale (a standardized tool for assessing the quality of evidence from non-randomized epidemiological studies. The average NOS score was 8.17, “indicative of high methodological quality.”
The papers included in the metanalysis examined a wide range of outcomes and endpoints: 36 studies looked at adverse birth outcomes (stillbirth, low birth weight, birth defects, etc); 8 papers covered infant or toddler cognition and neurodevelopment; 4 looked at child behavioral and psychological factors; 11 covered early childhood neurocognitive function, and 4 looked at early childhood behavior.
The data represented women and children from 7 countries: the US, Canada, Australia, the Netherlands, Norway, France, and Italy. Some studies utilized very large national databases. In total, this metanalysis represents 12,901,376 participants, 102,835 of whom were cannabis-exposed.
Adverse Birth Outcomes
The metanalysis led to some troubling conclusions:
- Higher Risk of Preterm Birth: Intrauterine cannabis exposure markedly raised the risk of preterm delivery; the odds ratio was 1.68. This finding was based on analysis of 20 studies looking at this particular question.
- Higher Incidence of Low Birth Weight: Maternal cannabis use during pregnancy conferred a shocking 2.6-fold increased risk of delivering a low birth weight child. That number is based on 18 studies assessing this risk.
- More NICU Admissions: Similarly, the odds ratio for NICU admission was 2.51 for neonates born to mothers who’d used cannabis during pregnancy compared with those born to mothers who did not. Ten studies in the metanalysis included data on NICU admissions.
“Our meta-analysis suggests that infants prenatally exposed to cannabis are more likely to be born preterm, with a low birth weight, and require NICU admission than infants without cannabis exposure. However, cannabis-exposed infants are not at greater risk of a birth defect or death within a year, including sudden unexpected infant death,” wrote Sorkhou and her co-authors (Sorkhou M, et al. Addiction. 2023).
Multiple Mechanisms
There are a number of mechanisms by which THC from maternal cannabis use could potentially impact fetal or early childhood neurodevelopment.
The compound binds to CB1 receptors, which appear very early in the fetal brain. They’re identifiable during the first trimester in the white matter, the hippocampus, and the prefrontal cortex. CB1 receptors are involved in proliferation, migration, and synapse development of neural progenitor cells.
Sorkhou and colleagues also point out that in utero cannabis exposure reduces tyrosine hydroxylase activity. This is the rate-limiting enzyme in dopamine synthesis, which means there could be changes in the densities of dopaminergic neurons, potentially increasing the propensity for substance abuse, and even schizophrenia. Fetal cannabis exposure can also alter expression and activity of opioid receptors, which play a role in motivation, mood and pain perception.
The impact of perinatal cannabis exposure can be longstanding and far reaching, say Sorkhou and her coauthors. One study they looked at showed atypical frontal cortex thickness in a group 6–8-year-olds who’d been exposed in utero (El Marroun H, et al. Biolog Psych. 2015).
Another showed diminished connectivity between the insula, cerebellum, prefrontal cortex and striatum in infants exposed to cannabis via maternal use (Grewen K, et al. Front Hum Neurosci. 2015).
“These early differences may adversely impact subsequent development of inhibitory control networks, and contribute to behavioral and cognitive deficits reported in children with prenatal cannabis exposure,” Sorkhou points out.
Impact on Cognitive Function
Mechanisms aside, the actual data on impact of in utero cannabis exposure on childrens’ cognitive function and behavior was less clear cut than the findings on adverse birth outcomes.
Sorkhou and colleagues saw some evidence that it is associated with diminished ability to pay attention, and also with an increased tendency toward externalization problems (disruptive behavior, ADHD, impulsivity, etc). But the data did not show correlations between cannabis and other behavioral or cognitive domains.
But they point out that the cognitive function studies they looked at were quite heterogenous in terms of cognitive outcomes measured. This made it difficult to synthesize and quantify the findings in terms of odds ratios and risk assessments.
In utero cannabis exposure reduces tyrosine hydroxylase activity. This is the rate-limiting enzyme in dopamine synthesis, which means there could be changes in the densities of dopaminergic neurons, potentially increasing the propensity for substance abuse, and even schizophrenia.
Impaired Attention
They note that 4 of the 10 studies looking at cognitive measures in young children showed impairments, primarily in attention and verbal reasoning skill.
One of these, by Lidush Goldschmidt and colleagues at the University of Pittsburgh, used the Stanford-Binet Intelligence Scales to evaluate cognitive function in a cohort of 648 school age children, found “a significant nonlinear relationship between marijuana exposure and child intelligence.” Daily maternal cannabis use during the first trimester correlated with lower verbal reasoning scores, and heavy use during the second trimester was predictive of short-term memory deficits as well as lower quantitative intelligence scores in the children (Goldschmidt L, et al. J Am Acad Child Adol Psych. 2008).
Julia Noland and colleagues at Vanderbilt University assessed 330 children between the ages of 6 and 9, using the Continuous Performance Test (CPT). After controlling for a host of potentially confounding variables, they observed a clear dose-dependent inverse relationship between maternal cannabis use during pregnancy, and childrens’ CPT scores.
The mothers who used cannabis once weekly or more, as confirmed by meconium analysis, were far more likely than non-users to have children with impaired attention. This study also included data on the impact of in utero exposure to cocaine and other drugs (Noland JS, et al. Neurotoxicol Teratol. 2005).
A Role in Autism?
In utero cannabis exposure may also predispose children to autism, according to a 2020 study by researchers at the Ottowa Hospital Research Institute. Using records from the Ontario Provincial Birth Registry, they calculated the incidence of autism spectrum disorders in children born during the five-year period between April 2007 and April 2012.
Among children whose mothers did not use cannabis while pregnant, the incidence of autism was 2.42 cases per 1,000. In contrast, the number was 4.0 in a matched cohort of kids whose mothers did use marijuana during pregnancy. The adjusted hazard ratio was 1.5 (Corsi DJ, et al. Nature Medicine. 2020).
Conflicting Conclusions
All this being said, the Sorkhou paper points out that 6 out of the 10 cognitive function studies showed no detrimental effect from maternal cannabis use.
The Noland group studied a cohort of 316 four-year-old kids, and found no relationship between maternal marijuana use and impairment of executive function. Prenatal exposure to alcohol, on the other hand, did correlate with impairment (Noland JS. Alcohol Clin Exper Res. 2003).
More recently, Aaron Murnan and colleagues compared a group of 14 four-year-old kids who had been exposed to cannabis in utero, with a matched control group. They found no differences between the two groups in terms of attention, self-control, executive function, or memory (Murnan AW, et al. J Applied Dev Psychol. 2021).
While the data on childhood behavior and cognitive function are somewhat equivocal, the Sorkhou group’s observations about the risk of premature birth, low birthweight, and NICU admissions are alarming, especially given the current stats on cannabis use among reproductive age women.
Young Moms & Marijuana
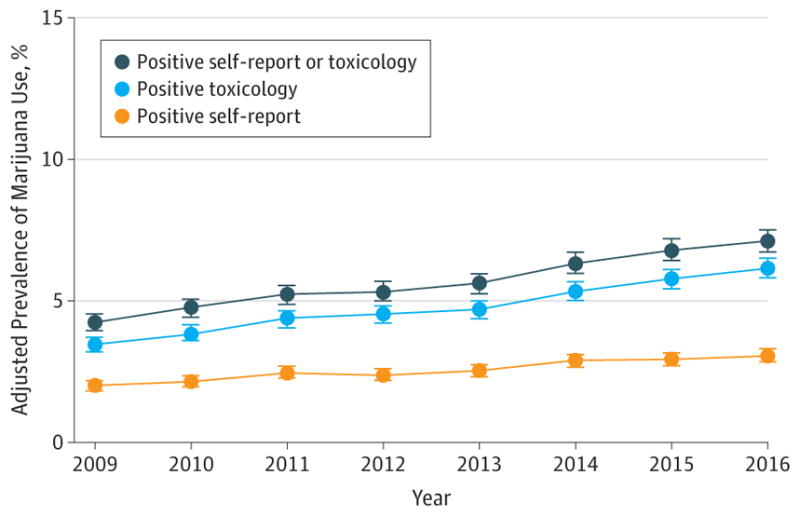
A 2019 paper by researchers at the NIH’s National Institute of Drug Abuse, based on self-reports of cannabis use from 467,100 women between the ages of 12 and 44, showed that prevalence of past-month cannabis use during pregnancy doubled from 3.4% to 7% between the years 2002 and 2017. Among the women who reported using cannabis, first trimester use rose from 5.7% to 12.1% during that 15-year span. The number of daily users rose from 0.9% to 3.4% (Volkow ND, et al. JAMA. 2019).
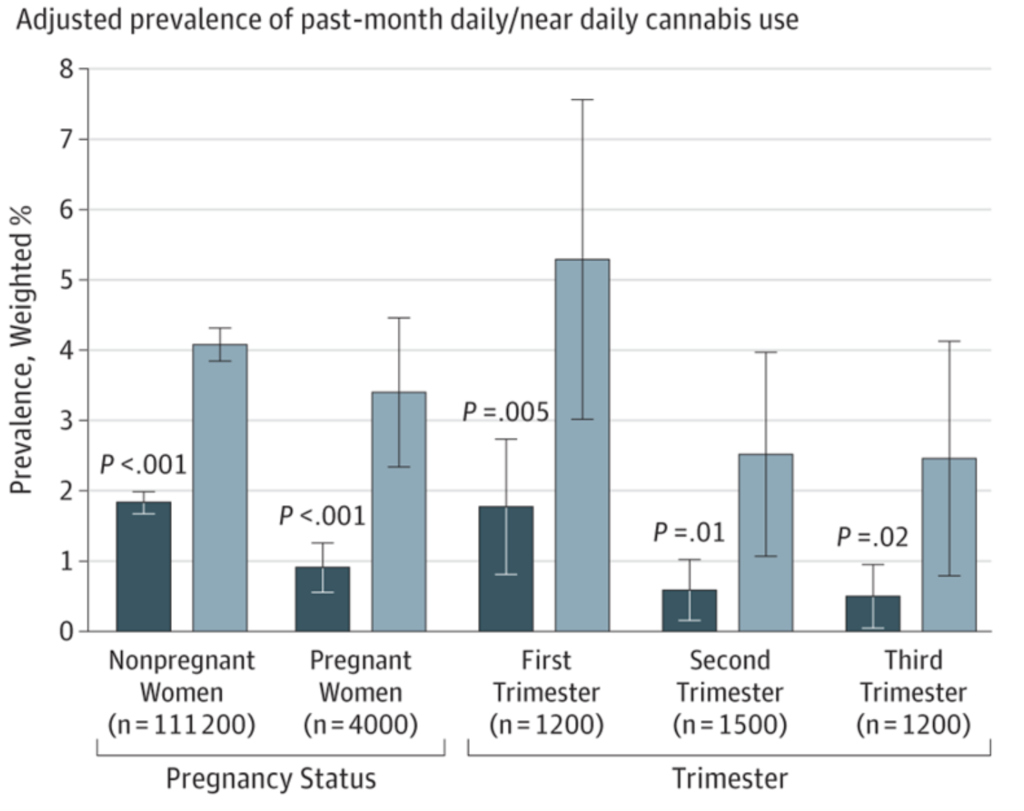
Some studies have suggested that nearly one in ten pregnant women in the US and Canada has used cannabis at some point during pregnancy.
And those figures are probably underestimates. Kaiser-Permanente researchers studied records from over 300,000 pregnant women between the ages of 12 and 24, comparing self-reported incidence of cannabis use against actual toxicological data from prenatal visits. The prevalence based on toxicology was roughly two times higher than it was based on self-reporting alone (Young-Wolff KC, et al. JAMA. 2017).
In other words, patients may not be entirely forthcoming or honest about their actual use of cannabis, because they fear judgment or reproach from their practitioners or, in some cases, because they fear losing custody of their children.
Mitigation of Nausea
Many women are using cannabis to mitigate the nausea, pain, and discomfort they experience during pregnancy. Kelly Young-Wolff and her group at Kaiser-Permanente found that use was considerably higher among women formally diagnosed with nausea and vomiting in pregnancy (NVP) compared with those who did not experience NVP (11% vs 5.8%).
Rachel Westfall and colleagues at the University of Victoria, British Columbia, surveyed a group of 51 women who used cannabis throughout their pregnancies. The majority (77%) said they used the herb to manage nausea. And 50% or more said they were using it to reduce chronic pain, help with sleep, reduce anxiety, manage depression, or treat fatigue (Westfall RE, et al. Compl Ther Clin Pract. 2005.)
Given the correlations between cannabis use and adverse pregnancy outcomes, it would be wise for clinicians to learn about other treatments to help women reduce the burden of nausea, pain, and insomnia during pregnancy.
Though cannabis use may be generally safe, and potentially therapeutic for women who are not pregnant, it can significantly raise the risk of serious neonatal problems, and may have detrimental effects on some aspects of childhood brain development.
Methodologic Limitations
As with all metanalyses and systematic reviews, the report by Sorkhou and colleagues was hindered by a number of methodological challenges and limitations which the authors readily admit.
The studies they reviewed varied widely in terms of the fetal and childhood outcomes they measured, and the methods used to make those assessments.
Further, they varied widely in terms of populations studied, and methods for assessing maternal cannabis use. Some studies included urine, saliva, or meconium testing for presence of cannabis metabolites, but others relied solely on the womens’ self-reporting of use. Some included a lot of data about timing, frequency, and level of marijuana consumption, while others were based on simplistic “Yes/No” questions about use.
In general, though, the 57 studies included in the analysis were large—some were massive—and of generally good statistical quality.
Bearing in mind the aforementioned caveats, the findings reported by Sorkhou et al raise very important issues:
- Cannabis use among pregnant women is more common than many people would imagine, and many others would like to admit.
- Some women are using cannabis to quell the nausea, pain, sleeplessness and other discomforts they experience during pregnancy.
- Though cannabis use may be generally safe, and potentially therapeutic for women who are not pregnant, it can significantly raise the risk of serious neonatal problems, and may have detrimental effects on some aspects of childhood brain development.
Keep these things in mind when working with pregnant patients, and learn how to ask about cannabis use in a non-judgmental way.
“Explore patients’ reasons for cannabis use, as many pregnant patients report use to provide relief from pre-existing conditions or pregnancy-related symptoms, including nausea, chronic pain and psychiatric disorders,” says Maryam Sorkhou. “When cannabis is being used for these purposes, providers should discuss alternative solutions, including safer substitutions. Pregnant women using cannabis therapeutically will also require guidance regarding when and how to resume use postpartum.”
END







