In my search for strategies that can limit Covid-19, I’ve discovered a potential role for non-toxic anti-viral nasal sprays. You may want to consider them for yourself and your patients, in situations in which you or they are potentially exposed to the virus, including work, travel, school or social encounters.
The mucosal lining of the nose is the main portal of entry for SARS-CoV-2. Mucosal cells contain the highest concentration of the factors needed for the virus to enter. The nose thus acts like an incubator in which the virus multiplies, and from which it is inhaled into the lungs (Hou YJ, et al. Cell, 2020) or directly enters the brain.
A laboratory study of hamsters found that the Omicron variant achieves the same concentration in the nose as does the delta variant, even though the levels in lungs are much lower (Diamond M, et al. Research Square, 2021).
Therefore, it makes sense that preventing or limiting viral entry into the nose has the potential to prevent or reduce infiltration of the lungs and thus systemic disease. There’s some evidence that intranasal sprays can help.
The mucosal lining of the nose is the main portal of entry for SARS-CoV-2. Therefore, it makes sense that preventing or limiting viral entry into the nose has the potential to prevent or reduce infiltration of the lungs and thus systemic disease.
As proof of concept, early research on ferrets indicated that a spray containing a lipopeptide that mimics amino acids on the viral spike protein, could effectively block nasal entry and prevent viral transmission.
Israeli researchers later showed that Taffix, an intranasal powder containing hypromellose and citric acid, gave a four-fold reduction in person-to-person transmission among congregants for Rosh Hashanah (Jewish New Year) religious services, which were considered to be super-spreader events (Klang S, et al. Expert Rev Anti Infect Ther. 2021).
Currently, there are 15 different nasal sprays under development around the world, that employ various compounds, among them hypromellose, carragelose, nitric oxide, and heparin, all of which can block viral entry via different mechanisms. Unfortunately, none of them are widely available in the US.
Intranasal Heparin
A more accessible option is intranasal heparin. This is extremely promising as a modality for preventing entry of SARS-CoV-2 into the nasopharynx and lungs. Though there are no ready-made commercial products yet on the market, it is something one can order from compounding pharmacies.
Most commonly prescribed as an anticoagulant, heparin is a derivative of a natural substance called heparan sulfate, which is part of a cellular coating called the glycocalyx, found on the membranes of cells throughout the body.
Heparan plays a key role in the entry of SARS-CoV-2 into human cells, a multistep process in which the viral spike proteins attach to angiotensin-converting enzyme (ACE)-2, embedded in the membranes of respiratory mucosal cells. Heparan on the cell membrane holds the viral spike protein in place, enabling its attachment to ACE-2 (Partridge LJ, et al. BioRxiv. 2020).
Blocking Viral Entry
Without this initial linkage to heparan, the virus is not able to find the ACE2 molecules. (Martino C, et al. BioRxiv. 2020). That’s where heparin comes in handy.
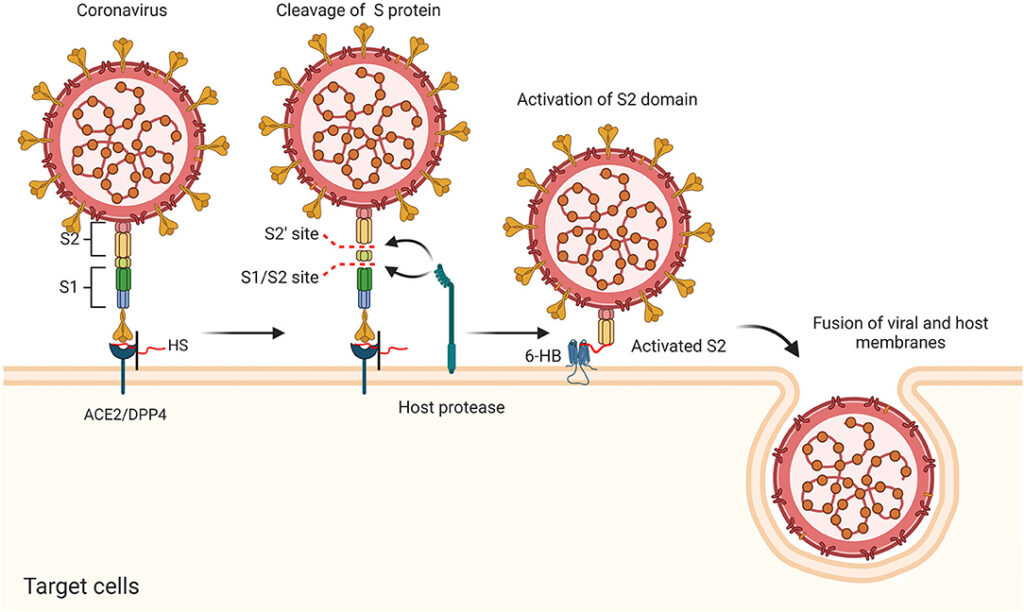
Free heparin, administered as a medication, can act as a decoy, attaching to the viral spike protein and rendering it unable to attach to heparan (Clausan TM, et al. Cell. 2020). This decoy binding is very tight, irreversible, and occurs at extremely low concentrations of heparin (Kim SY, et al. Antiviral Res. 2020).
Injected heparin is widely used to treat or prevent blood clots in hospitalized patients with COVID-19. Inhaled heparin, given at high doses by nebulizer, has been used to treat acute respiratory distress syndrome (ARDS). The purpose of inhaled heparin is to prevent widespread clotting in the small blood vessels of the lungs, but it has no significant effect on systemic coagulation, even at high doses (Van Haren FMP, et al. Crit Care. 2020)
At the low dose in this nasal spray, heparin does not kill the virus; but it prevents viral attachment to the lining of the nose. It is safe, simple, and stable.
The goal of nasal heparin is simply to prevent attachment of the SARS-CoV-2 spike protein to ACE-2, thus neutralizing the virus. The dose needed for this effect is much lower than that needed to prevent blood clots. This is easy to attain without risk of systemic anticoagulant effects.
Low Dose, Low Risk
In July 2020 I designed a nasal spray containing low dose heparin dissolved in salt water. I have it made by compounding pharmacies.
The heparin concentration in this spray is 10 units/ml, which should be more than enough to saturate the virus, even when diluted by nasal secretions. The entire spray bottle contains only 300 units of heparin.
Because of the heightened transmissibility of the Omicron variant, and after reviewing safety data published by researchers at the University of Mississippi, I recently recommended an increase in concentration to 1000 units/ml. By comparison, clinicians treating hospitalized COVID patients with inhaled heparin are giving 25,000 units each time.
At the dose used in this nasal spray, heparin does not kill the virus; but it prevents viral attachment to the lining of the nose. It is safe, simple, and stable.
This formula is intended for use as needed for potential exposure, but it is safe enough to be taken daily for extended periods of time. It should be sprayed into each nostril soon before and soon after a potential exposure to COVID, and may be repeated 4 hours after the exposure. It can be used daily, every 4 hours, if you have continuous or repeated exposures. The solution has a shelf life at room temperature of about a year, but is cleared by the pharmacies for shorter periods of time, to insure sterility.
Free heparin, administered as a medication, can act as a decoy, attaching to the viral spike protein rendering them unable to attach to the heparan on the cell membranes.
Currently, two pharmacies are compounding the formula I developed: The Healthy Choice pharmacy uses a preservative to extend storage safety; CareFirst’s formulation is preservative-free. Each bottle contains 300 sprays. I have no financial interest in these sprays. They are only available with a doctor’s prescription.
Note that commercially available heparin is derived from pork intestine, so people who are allergic to or otherwise averse to pork should not use these sprays. Pseudo-allergic reactions to heparin may also occur. Similarly, those who are prone to nosebleeds should not use intranasal heparin, and it should be discontinued one day before any dental surgery or Ear, Nose, and Throat (ENT) procedure.
Because it has to cover only a small surface area, intranasal heparin spray provides a very low systemic dose, but a relatively high concentration in the nose. Generally, this is quite safe, though there may be a local anticoagulant effect, limited to the inside of your nose and perhaps your mouth. Anyone experiencing swelling or difficulty breathing after using this spray should discontinue immediately.
A research team in Australia is starting a clinical trial of a heparin nasal spray for prevention of COVID-19. In the words of these investigators, heparin “can rapidly wrap around the virus’s spike protein like a python, preventing it from infecting you or spreading the virus to others.”
They are testing a dose much higher than the one in the spray I designed, and have not seen adverse effects. Because of the high transmissibility of the Omicron variant, I have asked the two pharmacies making my formula to increase the concentration in the sprays. Note that I have no financial interest in these intranasal heparin sprays.
Researchers at the University of Mississippi and Rensselaer Polytechnic institute also hope to create a commercial prescription spray based on heparin.
But you do not need to wait for their spray to be approved; compounding pharmacies can make them for you and your patients.
Other Options
As mentioned at the beginning of this article, there are several anti-viral nasal sprays now commercially available in other countries. They include:
Carragelose (Marinomed Biotech, Vienna): Carragelose is a trademarked form of iota-carrageenan, derived from red marine algae. It produces a non-specific coating of the nasal lining, and may have some general anti-viral activity.
In clinical trials, Carragelose shortened the duration of upper respiratory infection (Ludwig M, et al. Respiratory Research. 2013), but there are not yet any studies to establish its preventive benefits or the safety of continuous preventive use.
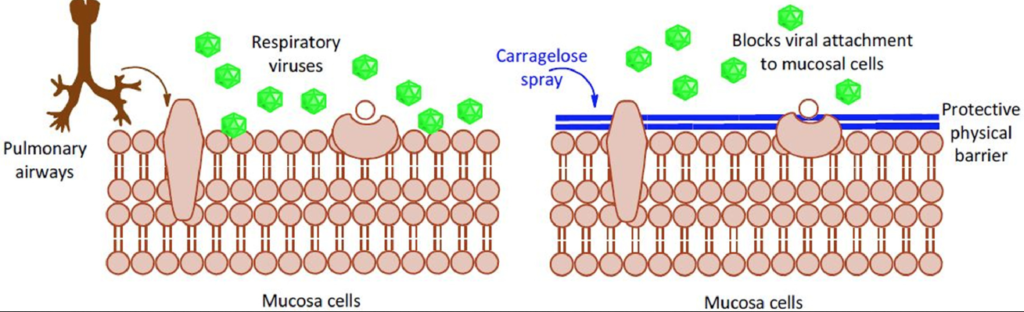
There is evidence from a study of human lung cell cultures that the substance has antiviral activity against the Alpha, Beta, Gamma, and Delta variants of SARS-CoV-2 (Froba M, et al. Int J Mol Sci. 2021). This follows several earlier in vitro studies suggesting antiviral activity against SARS-CoV-2. But the effect has not yet been confirmed in actual human clinical studies.
That said, an Argentine study of a generic iota-carrageenan product combined with the drug ivermectin as a mouthwash did show significant benefits in preventing COVID-19 among health care workers.
Carragelose nasal sprays, throat sprays, and lozenges are commercially available in 29 countries for treatment of common respiratory viral infections. It is not readily available in the US. Marinomed Biotech says human trials looking at Carragelose for COVID-19 prevention are underway in the UK and Austria.

Taffix (Nasus Pharma, Tel Aviv): As noted earlier, Taffix is a nasal powder containing hypromellose and citric acid, commercially available in Israel, the UK, and Europe. Taffix creates a diffuse coating of the nasal lining. It’s acidic pH of 3.5 is allegedly anti-viral, though SARS-CoV-2 is stable at pH as low as 3.0.
In a non-randomized clinical trial, Taffix users had a 78% reduction in incidence of COVID-19 compared to non-users following prolonged communal prayer.
VirX / Enovid (SaNOtize): These are nitric oxide nasal sprays developed by a Canadian research and development company called SaNOtize.VirX and Enovid are identical; the names vary depending on the countries in which they’re sold. Intranasal NO has been shown to completely eradicate SARS-CoV-2 in the nose after 24 hours of administration.

SaNOtize describes this product as “like a hand sanitizer for the nose, creating a physical barrier in the nasal passages that stops viruses.” It should be used every 4 hours for a minimum of 24 hours after a known exposure.
The company is promoting it as a potential early stage treatment for COVID-19, and it was the subject of an 80-patient, UK-based clinical trial that showed marked reduction in viral load, but did not look at clinical endpoints (Winchester S, et al. J Infect. 2021)
It could be helpful for people within one to five days of having been exposed to someone known to have COVID-19. Though not widely available in the US, Enovid can be purchased via IsraelPharm, an online pharmacy that delivers worldwide within 7-10 days.
END
Leo Galland, MD, is an internist in New York City, specializes in the evaluation and treatment of patients of all ages with complex chronic disorders. He is internationally recognized for developing innovative nutritional therapies to treat autoimmune, inflammatory, allergic, infectious and gastrointestinal disorders and has described his work in numerous scientific articles and textbook chapters.
A graduate of Harvard University and New York University School of Medicine, Dr. Galland is board-certified in internal medicine. He is listed in Leading Physicians of the World and America’s Top Doctors. In 2017, Dr. Galland was awarded the Albert Nelson Marquis Lifetime Achievement Award by Marquis Who’s Who. Dr. Galland is a pioneer in studying the impact of the gut microbiome and intestinal permeability (“leaky gut”) on health and disease. He is the author of several ground-breaking popular books including The Four Pillars of Healing (1997), Power Healing (1998) and The Allergy Solution (2016).
Contact Dr. Galland at: info@galland-health.com







