
Antimicrobial resistance is just the tip of the iceberg of adverse health consequences caused by antibiotic overuse, according to Martin J. Blaser, MD, director of the Rutgers University Center for Advanced Biotechnology and Medicine.
The less obvious—and therefore more dangerous—part of the iceberg, he says, is the epidemic of chronic inflammatory diseases driven in large part by antibiotic-associated microbiome perturbations. He contends that this hidden problem has potential to shipwreck the nation’s already tattered healthcare systems.
A pioneer in the field of microbiome research, Dr. Blaser holds that antibiotic overuse is a major factor in the epidemics of diabetes, obesity, asthma, food allergies, irritable bowel disease, and even neurological and behavioral conditions like ADHD and autism.
“We have 10 different diseases all going up at the same time. Do they have 10 different causes? Perhaps there is one causal factor that underlies them all: a change in the human microbiome,” he said in a webinar entitled, Antibiotics at the Crossroads, sponsored by Howaru probiotics.
While there are many factors influencing disease development, the ubiquitous use of antibiotics is a big one, says Blaser. Overuse, which is prevalent worldwide, is the result of longstanding practice habits combined with unrealistic patient expectations.
A Key Risk Factor
“Antibiotics entered routine medical practice around 75 years ago. They’ve saved innumerable lives and revolutionized every aspect of medicine. But doctors and other practitioners are using them more and more. And that has had unintended consequences.”
Worldwide consumption of antibiotics has topped 73 billion doses per year, Blaser says. “That’s roughly 10 pills for every man woman and child on earth.”
More than half of all pregnant women in the US get antibiotics at some point during pregnancy, either for treatment of a specific infection or as “prophylaxis.” That’s a problem because it disrupts the intergenerational handoff of the microbiome.
–Msrtin Blaser, MD
The Centers for Disease Control estimated in 2013 that Americans took over 258 million courses of antibiotics annually (Hicks LA, et al. N Engl J Med. 2013). That’s about 833 courses per 1,000 people. In simpler terms, five courses for every six people.

Overuse is particularly common in children, says Blaser. By the time an average American child is two years old, she’s been exposed to antibiotics twice or even thrice. By the time she’s 10, she’s had 11 antibiotic treatments.
More than half of all pregnant women in the US get antibiotics at some point during pregnancy, either for treatment of a specific infection or as “prophylaxis.”
That’s a problem because it disrupts what Blaser called “the intergenerational handoff of the microbiome.” Intensive exposure during pregnancy and early childhood perturbs the microbiome at critical stages in its development, which has metabolic consequences.
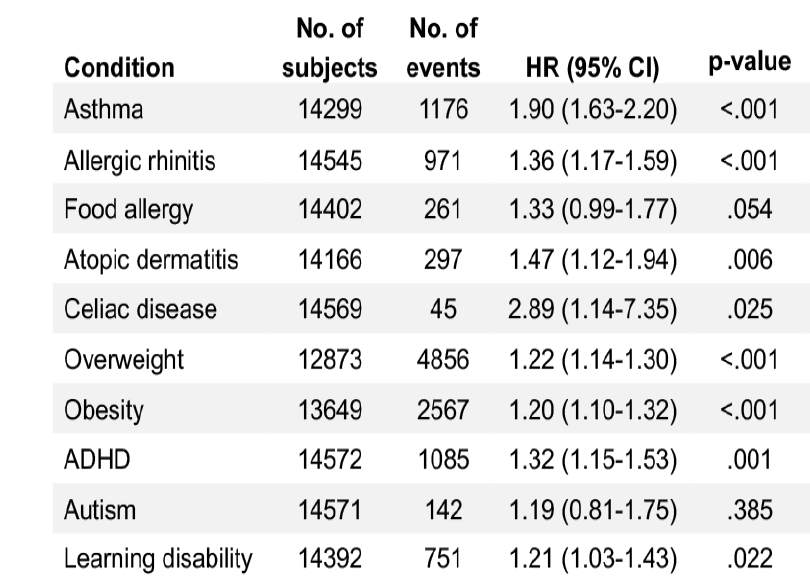
Blaser stressed that all ten of the most common childhood health conditions showed increased hazard ratios associated with antibiotic exposure in the first 2 years of life: Asthma, allergic rhinitis, food allergies, atopic dermatitis, celiac disease, overweight, obesity, ADHD, autism, and learning disabilities. Celiac showed a remarkable 3-fold increased hazard ratio.
Cesarean births and formula feeding have also disrupted the intergenerational microbiome transfer. But by far the biggest impact is from the broad use of antibiotic drugs, citing a 2012 paper he published in Nature Reviews Genetics.
Antibiotic-Induced Obesity
Physicians have been reluctant to accept the notion that antibiotics drive human weight gain, but it’s a phenomenon well-known and widely embraced in animal agriculture.
Shortly after the first antibiotics became available, farmers recognized that these drugs were growth promoters. Today, antibiotics are fed to livestock in unimaginable quantities, not just to fight pathogens, but primarily to fatten the animals.
“They feed the animals antibiotics to make them grow faster,” Dr. Blaser explained.
“It works from chickens to cows, which is a wide swath of vertebrate evolution. It works with virtually any antibacterial that has been tried regardless of chemical structure, class, target, or spectrum. Antivirals do not work, and antifungals do not work.”
Studies of swine show that the earlier in an animal’s life the antibiotics begin, the bigger the effect on growth rate and feed-efficiency (conversion of calories into body mass).
There is some evidence that humans also gain weight after taking antibiotics. Several observational studies show that people on broad or narrow-spectrum antibiotics are more likely to gain weight than untreated people (Leong KSW, et al. Clin Endocrinol. 2018).
Physicians have been reluctant to accept the notion that antibiotics drive human weight gain, but it’s a phenomenon well-known and widely embraced in animal agriculture.
In a Danish interventional study, 12 lean healthy men treated for four days with a combination of vancomycin, gentamicin, and meropenem showed an average 1.3 kg increase in body mass following the treatment (Mikkelsen KH et al. Diabetes Obesity Metabolism. 2016).
There are seven solid epidemiological studies showing that early life exposure correlates strongly with increased body mass index (BMI) and higher prevalence of obesity in children. The associations are more pronounced among males, among kids treated within the first 6 months of life, and among those exposed to 3 or more courses.
There are also 10 randomized controlled trials suggesting antibiotic use in prepubertal children leads to weight gain. But it is difficult to draw definitive conclusions because most studies did not account for the possibility that the observed weight gain was due to resolution of the infections for which the drugs were prescribed.
Xiaoqing Shao and colleagues at Fudan University, Shanghai, analyzed data from 15 studies representing a total of more than 445,000 children, and found that antibiotic exposure in the first years of life confers a 23% increased risk of being overweight later in childhood (Shao X, et al. Frontiers Endocrinol. 2017).

The authors report “an obvious dose–response relationship between antibiotic exposure in early life and childhood adiposity, with a 7% increment in the risk of overweight and a 6% increment in the risk of obesity for each additional course of antibiotic exposure.”
There are grounds for critiquing Shao’s metanalysis; it relies on observational studies and data based on mental recall of antibiotic use, and it fails to adjust for genetic predispositions, diet variances, and other potentially confounding variables. But the findings cannot be entirely dismissed.
Of course, correlation is not synonymous with causation. One of Blaser’s major scientific contributions has been to identify the physiological mechanisms linking antibiotic exposure with changes in the microbiome, and alterations in metabolism.
Multiple Mechanisms
He and his team have shown that antibiotics can influence weight gain via multiple mechanisms: they increase the ability of some gut bacteria to extract energy from indigestible polysaccharides; they decimate populations of gut commensals that are protective against obesity; and they alter hepatic lipogenesis and metabolic signaling. By killing off “friendly” flora they squelch intestinal immunity.
In his excellent 2015 review entitled Antibiotics in Early Life and Obesity, Blaser and his colleague Laura Cox report that: “When given early in life, agents that disrupt microbiota composition and consequently its metabolic activity, can influence body mass of the host by either promoting weight gain or stunting growth, which is consistent with effects of the microbiota on development” (Blaser MJ, Cox LM. Nat Rev Endocrinol. 2015).
Researchers at Penn State University showed that in mice, a 20% increase in Firmicutes and a corresponding 20% decrease in Bacteroides leads to an increase of 150 kcal per day, on average, released from normally indigestible polysaccharides. This is equivalent to a 4%-10% increase in daily calories (Harris K, et al. J Obesity. 2012). Further, release of certain short chain fatty acids can modulate the secretion of gut hormones such as peptide YY and glugagon-like peptide, which alter sensation of satiety.
Back in 2012, Ilseung Cho, a post-doctoral fellow in Blaser’s lab, studied body fat as a percentage of total body mass in 10-week old mice. Among control mice not receiving antibiotics, fat represented roughly 23% of total mass. But it was 32% among mice given antibiotics– penicillin, vancomycin, the combo of both, or tetracycline at doses equal to the midpoint of the FDA approved dose-range for livestock (Cho I et al. Nature 2012).
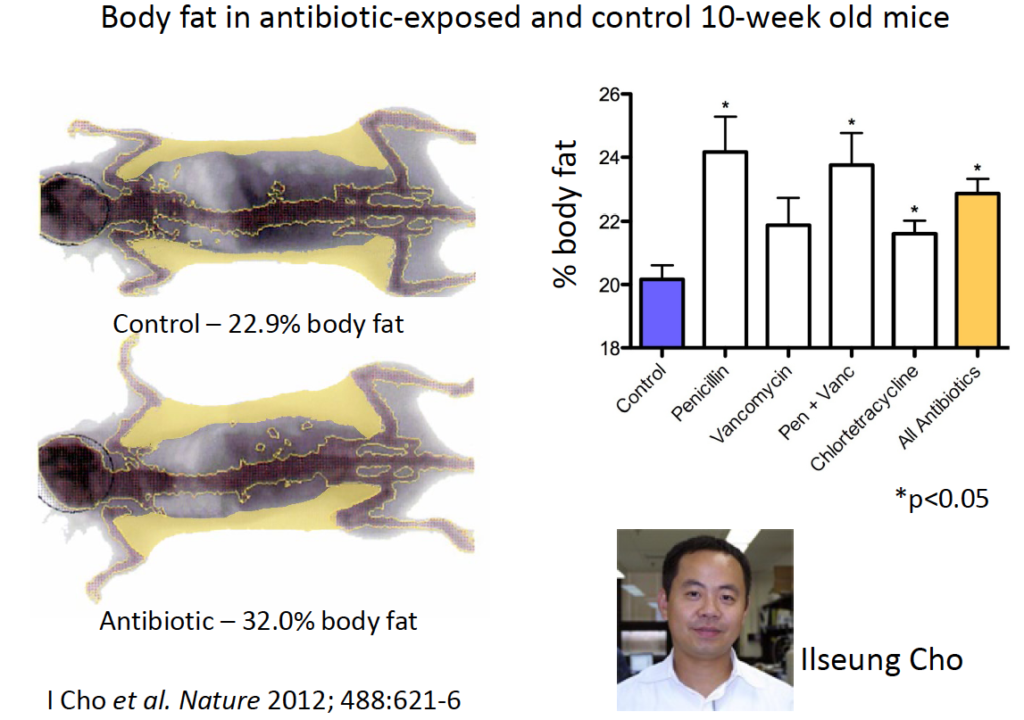
In 2014, Laurie Cox showed that antibiotic exposure during the first 4 weeks of life induced significant morphometric changes in mice. Total body mass, lean body mass, and fat mass were all increased in antibiotic-exposed animals relative to unexposed controls. These long-lasting increases correlated with level and duration of antibiotic exposure. (Cox L, Cell. 2014).
Transferrable Trait
In light of decades of agricultural experience, these findings were not entirely surprising.
What was shocking was the discovery that this tendency toward weight gain is transferrable to animals that had never ingested antibiotics.
“The metabolic signal is in the microbiome,”
–Martin Blaser, MD
“We did fecal transplants. We gave mice antibiotics, or not, and then harvested microbiome from their cecums and gave these to germ-free mice with no microbiomes of their own. The recipient mice never saw an antibiotic. Yet those receiving microbiome transplants from antibiotic-exposed mice put on more weight than those getting transplants from non-exposed animals,” Dr. Blaser says. “There was no change in lean mass, but there was a change in fat mass, in 5 weeks.”
“The metabolic signal is in the microbiome,” he added.
Even a transient effect on the microbiome can have a lifelong metabolic effect in the mice, if it happened at a critical early point in development. He contends that the situation in humans is not so different.
Immunological Effects
Antibiotics alter intestinal Th17 cell populations, says Dr. Blaser. “Whether you look at small or large intestine, in every case the numbers are down in the mice receiving antibiotics. The drugs diminish the number of these important immune cells. This is a very reproducible finding.”
As with the weight gain trait, this immune system alteration is also transferrable.
Antibiotic-naïve mice who got fecal transplants from exposed mice quickly show Th17 and antimicrobial peptide profiles resembling those of mice that had received antibiotics.
“This is evidence that not only are there changes in metabolism there’s a change in immunity from the perturbed microbiome. This is direct causal evidence.”
A Public Health Threat
The consequences of antibiotic-induced microbial disruption can be transient or long term, they can be situational, they can be senescent, or generational. They can also be immunologic, metabolic, neoplastic or maternal. There’s evidence for all of this, Dr. Blaser says.
All ten of the most common childhood health conditions showed increased hazard ratios associated with antibiotic exposure in the first 2 years of life.
He believes the medical community needs to take these phenomena seriously, as a major public health issue.
Consider a recent Mayo Clinic study. Researchers looked at early life antibiotic exposure among more than 14,000 kids in Olmstead County, MN, over the 13 year period between 2003 and 2016. Within this cohort, 10,220 (70%) got at least one course of antibiotics within the first 2 years of life (penicillins 63.9%, cephalosporins 23.3%, macrolides 25.6%). Only 4,352 kids were not treated with antibiotics during early childhood.
“If you look at Kaplan Meier curve of asthma development, the prevalence was nearly twice as high among girls and boys who got antibiotics, compared with those who did not. And it was higher in males than in females,” Blaser says (Aversa Z et al. Mayo Clin Proc. 2021).
Ten years ago, Danish researchers looking at prevalence of irritable bowel disease in children, showed very clearly that the more antibiotics kids get the greater their odds of developing IBD (Hviid et al. Gut. 2011).
Dr. Blaser and his team have studied this phenomenon in wild type mice versus IL-10 deficient mice that spontaneously develop an IBD-like syndrome as a result of this genetic alteration
When the normal, wild type mice are exposed to antibiotics they develop lots of colonic inflammation that resembles the patterns seen in the IL-10 deficient mice. And again, this inflammatory pattern could be passed from exposed to unexposed mice via fecal transplant. Further, it was also intergenerational. Non-exposed babies of exposed mothers with the IBD-like disorder showed the colonic inflammation (Schulfer et al Nature Microbiol 2017).
“It is entirely microbiome-based, and it is transferrable across generations,” Blaser says. “This has profound implications.”
He is calling on practitioners and patients alike to re-set their attitudes and habits regarding antibiotics.
“We are killing our beneficial bacteria. To reverse course, we need to curtail all unnecessary use of antibiotics. But that alone will not suffice. That will just keep us where we are. To improve, we need to restore the healthy microbiota.”
The good news is that the icrobial perturbations by antibiotics, which drive a lot of autoimmune conditions, can be reversed and normal patterns can be restored, through targeted use of probiotics. Both the science and the clinical practice of this are in their earliest stages. Blaser hopes that it will accelerate as the decade progresses, and that probiotic considerations will begin at the earliest stages of life.
“My prediction is that in the medicine of the future, when doctors examine newborn babies they will examine not just the babies themselves, but their diapers. And they will ask, “Does this baby have the microbes that all babies should have? Do they have the appropriate personal microbes? If they don’t they can optimize health by administering supplements to reshape the microbiome to put these kids on the best path to health.”
We’re a long way from that right now, but there’s no question that the burgeoning field of microbiome research is affecting clinical practice.
By understanding the metabolic costs associated with antibiotic treatments, clinicians can factor them into the equations of clinical guidelines and decisions, to continue to support the prudent use of antibiotics while minimizing overuse and, hopefully, stemming at least some of the myriad chronic inflammatory diseases that now burden so many people.
END







