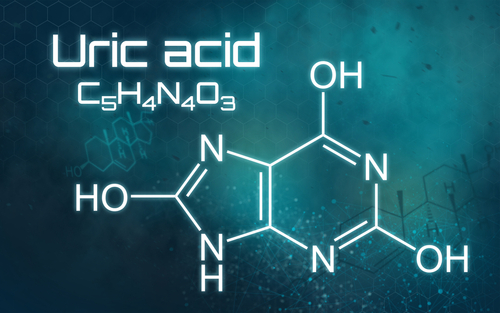
Conventional medical wisdom holds that uric acid is a harmless, inert form of metabolic waste, and most physicians were trained to see it as a “trivial, incidental byproduct of our normal biology.” But contemporary research reveals that this compound is “anything but meaningless or unworthy of our attention,” says neurologist David Perlmutter, MD.
In his latest book, Drop Acid: The Surprising New Science of Uric Acid – The Key to Losing Weight, Controlling Blood Sugar, and Achieving Extraordinary Health, Perlmutter contends that even modest uric acid elevations can have pernicious long-term consequences if left unchecked. People will do themselves a world of good by dropping acid — uric acid, that is.
Though UA testing is included in routine blood work, most people—unless they have gout or kidney stones– haven’t heard of it, and few can recite their UA levels the way they do with their lipid profiles.
Physicians themselves often ignore UA, or cast it aside as an “innocent bystander,” says Perlmutter. Most learned that UA is problematic only when it reaches the excessive levels that cause gout and kidney stones.
In reality, a UA rise is far more than just a risk factor for gout or kidney problems.
“Above 7 mg/dL is when UA begins to precipitate in the blood and form crystals. That obviously has relevance as it relates to gout and kidney stones. But the cardiovascular risk begins at a level of 5.5 mg/dL”

According to Perlmutter, elevated UA is a common thread linking a striking number of chronic conditions, including: obesity, insulin resistance, type 2 diabetes, nonalcoholic fatty liver disease (NAFLD), hypertension, coronary artery disease, stroke, and neurological disorders including Alzheimer’s.
Drop Acid draws on the work of Dr. Richard Johnson, a University of Colorado nephrologist who is among the world’s uric acid experts, and who guided Dr. Perlmutter in developing the book. It lays out a strong case that “asymptomatic hyperuricemia” driven largely by excessive fructose intake, precedes the development of elevated blood glucose, hypertension, dyslipidemia, weight gain, and chronic inflammation.

This is not a new concept. More than a century ago, a Scottish physician named Alexander Haig recognized a connection between elevated uric acid levels, and conditions like migraine, depression, epilepsy, diabetes, obesity and cardiovascular disease. Haig published a book on this in 1896, but his ideas failed to impress his medical contemporaries who insisted that uric acid was harmless except at extremely high levels.
Haig was obsessed with dietary purines as the main driver of uric acid elevation, and he advocated a highly restrictive “purine-free” diet that eliminated all meat, legumes, and many common vegetables.
Drop Acid, which Dr. Perlmutter wrote with longtime collaborator Kristin Loberg, has a different focus and takes a gentler approach. It states that in our current society, fructose is a far more important culprit than purines. The book’s “LUV (Lower Uric Values) Diet” emphasizes fructose reduction. While it does recommend reducing purine-rich meats and fish, the LUV Diet does not entirely eliminate them.
Uric acid “sits at the heart of regulatory mechanisms involved in our most fundamental processes of metabolism,” says Perlmutter, who is also author of Grain Brain, and several other best-sellers. Drop Acid urges practitioners to rethink everything they learned about UA, and to recognize that rising UA is a danger sign that we should no longer ignore.
Holistic Primary Care spoke with Dr. Perlmutter following the publication of Drop Acid. Here are some highlights from our interview:

HPC: As you note in Drop Acid, the standard medical definition of hyperuricemia includes only two adverse symptoms: gout and kidney issues. Given what we now know about UA, how do clinicians need to think differently about this compound?
DP: Over the years, what’s been so fundamentally important for me is understanding what makes a good metabolism go bad. What are the inputs for metabolic mayhem in humans? The reason for my deep wish to understand this is that metabolic disturbances underlie the most pervasive chronic degenerative conditions on the planet — which, according to the World Health Organization, represent the number one causes of death [globally]. It’s not COVID, it’s not anything infectious. It is the chronic degenerative conditions as a group — which are, at their core, related to disturbances of metabolism.
That’s why anything influencing metabolic health is interesting for me. As a neurologist, Alzheimer’s is fundamentally a manifestation of metabolic dysfunction. Alzheimer’s, a disease now affecting some 6 million Americans — which will triple in its prevalence by the year 2050 — is a metabolic issue.
Learning about the manifold mechanistic effects of elevated UA on disrupting metabolism offered a very new and exciting perspective. But beyond that, it’s a new and exciting potential tool to help bring people back into metabolic balance.That’s huge. That is absolutely the reason we’re in the game. To figure out anything that can be beneficial, that we can leverage to help people regain metabolic health.
“It’s time we put uric acid where it rightfully belongs, in terms of measuring it and following it over time, as we do with BMI, blood pressure, fasting blood sugar, and fasting blood insulin.”
There are a lot of new interventions focused on helping people understand and correct their metabolism, and this is certainly one of them. That’s what I found so attractive about uric acid. We tend to pigeonhole ideas and think, “That’s what these things do, end of story.” As in, “Testosterone is only a male hormone, or estrogen is only a female hormone.” Well, as a matter of fact, women have testosterone in their bodies, testosterone receptors in their brains. It’s very important to keep an open mind that some of the well-defined issues we studied in our education might be worthy of expansion.
We’ve learned that UA elevation is a powerful signal to our metabolism to do some important things: raising blood pressure, raising blood sugar, increasing production of body fat, reducing use of body fat for energy. And finally, reducing our metabolic demand by ratcheting down mitochondrial function.
This is significant, because in the context of our evolution, the elevation of UA proved to be an incredibly powerful survival mechanism. It would raise our blood sugar so we could power our intelligent brains when we had no food. It could give us a small advantage — a superpower, if you will — to survive during times of food scarcity by increasing our production of body fat. And part of that superpower was to reduce energy utilization by compromising mitochondrial function during times of caloric scarcity.
These days, however, those mechanisms remain operative and are in hyperdrive. They are on board 365 days a year, during a time when we have anything but caloric scarcity. We are basically triggering our bodies with this alarm signal — elevated UA — to prepare for a winter that never comes.
HPC: Once you’ve identified that a patient has elevated UA, how do you begin crafting an appropriate treatment plan? What role do dietary or lifestyle changes play, and are there cases where pharmaceutical interventions are more or less appropriate?
DP: First, as a general rule, we operate under the dictum of primum non nocere, meaning above all, do no harm. That tends to guide our hand to more of the lifestyle interventions on the front end, provided things are not too aggressively disturbed.
The first thing we generally do in an individual (with elevated UA) is to look at the diet. That means, first and foremost, reducing the consumption of fructose. Not necessarily fruit — but the fructose found in condiments, fruit juices, beverages of all kinds, soft drinks, etcetera.
Beyond that, we like to add in some nutritional supplements demonstrated in peer-reviewed science to be helpful in terms of lowering UA. These are things like quercetin and luteolin. They target the very same enzyme, xanthine oxidase, as allopurinol and febuxostat–the drugs used to treat gout.
Adding in 500 mg a day of quercetin and 100 mg of luteolin, along with dietary changes, often produces a pretty dramatic drop in UA in just a number of weeks.
Five Key Supplements for Reducing Uric Acid
- Quercetin: 500 mg/day
- Luteolin: 100 mg/day
- Docosahexaenoic acid (DHA): 1,000 mg/day
- Vitamin C: 500 mg/day
- Chlorella 1,200 mg/day
For a person who has resistant hyperuricemia where UA remains significantly elevated after reducing purines, alcohol, and substantially reducing fructose consumption, and after taking quercetin, luteolin, and maybe some tart cherry extract as well, one might well consider a pharmaceutical intervention like allopurinol, a very safe drug. But that is exceedingly rare.
HPC: If a patient with high uric acid has to choose which dietary adjustments to make first, what would you recommend prioritizing?
DP: Job number one, regardless of where a person lives and what they have access to, is to identify the hidden sources of fructose in the diet. In America, UA levels jumped from an average of 3.5 mg/dL in 1920 to 6 mg/dL in 1970, which perfectly correlates with our increased sugar consumption.
Ideally, we could get back to a diet that resembles what people were eating in the 1920s and 30s. It doesn’t have to be Paleo. And it’s not even so much what they were eating, but what they were not eating. And what they were not eating was sugar, as much.
What are the hidden sources of fructose? Fructose is 50% of table sugar. Sucrose is 50-50 fructose-glucose. Fructose is added now via high fructose corn syrup to more than 60% of all packaged foods at the grocery store. Because we like sweet.
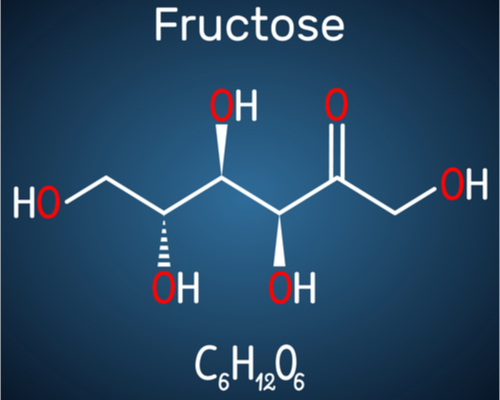
So, the top five dietary changes to make are: 1) reduce fructose. 2) reduce fructose. 3) reduce fructose. For numbers 4) and 5), we can talk about reducing foods high in purines, reducing the high levels of sodium that people might be eating, and certainly alcohol choices. But these days, it’s all about the fructose.
HPC: It sounds like a few relatively minor diet and lifestyle interventions can really pack a punch when it comes to reducing UA.
DP: That’s right. It’s time we put uric acid where it rightfully belongs, in terms of measuring it and following it over time, as we do with BMI, blood pressure, fasting blood sugar, and fasting blood insulin. We need to recognize how we affect it (UA) based upon our lifestyle choices. It’s really important, because elevated UA is upstream of all these other metabolic problems to which we’re so desperately responding.
When you look at the literature, it really hits home. For example, one study appeared in the journal Arthritis & Rheumatism in 2009. It was an eight-year study involving about 42,000 men and 49,000 women. The authors found that those individuals who had uric acid of 7 mg/dL or greater during the study had a 16% increased risk of all-cause mortality, or death from any cause. They also had a 39% increased risk of cardiovascular mortality, and a 35% increased risk of dying from an ischemic stroke. For every point (of UA) elevation over 7 mg/dL, there was an additional 8-13% increased risk of all-cause mortality (Chen, JH et al. Arthritis & Rheumatism. 2009. 61(2): 225-232).
Now, this is a correlative finding. It shows that there is a correlation between elevated UA and in this case, risk of death from any number of factors or diseases. But as we’ve begun to understand how elevated UA does its damage in the body, we would expect these numbers.
HPC: Speaking of the point at which UA does damage, what can you tell us about “normal” versus “optimal” uric acid ranges?
DP: “Normal” is defined basically as what is average in a large group of people. And on average today, 88% of American adults have at least one component of metabolic syndrome. That means only one in eight American adults is metabolically intact. A third of American adults is obese — not just overweight, but obese. And by 2030, which is only eight years away, that number will be 50%.
So, we’ve really got to get away from the notion of being part of the general crowd and wanting to be “normal.” Anyone reading HPC wants to know what’s best — and therefore, what is optimal.
As that relates to UA, the laboratory norms were developed in the context of gout. What we know is that above 7 mg/dL, UA begins to precipitate in the blood and form crystals. That obviously has relevance as it relates to gout and kidney stones. But the cardiovascular risk begins at a level of 5.5 mg/dL, not 7 mg/dL.
We begin seeing significant increases in terms of cardiovascular issues and other issues related to UA at 5.5 mg/dL. So, we really want to be at 5.5 mg/dL or lower. There, people can really seek to achieve longevity, a long healthspan, and importantly, disease resistance.
Now that we know what the lifestyle interventions are for lowering UA, it becomes yet another powerful tool in our tool box. Not as an end-all — it’s not as if you’re going to be totally at no risk for metabolic issues just because your UA level is normal and you’re eating appropriately. Everything works in an integrative way.
HPC: It’s remarkable how vast the role of UA really is. It’s so much more than a gout or kidney issue.
DP: It’s not your grandfather’s uric acid anymore. In the context of our ancestors, it was wonderful that we as humans had this series of mutations that removed uricase — the enzyme that would have broken down UA — from our bodies.
Genetic changes that began 15 million years ago in our primate ancestors compromised our ability to break down UA. Our UA levels as humans are four to five times higher than the UA levels of other mammals. That proved advantageous for almost our entire time on this planet. Not by making us fat, not by making us diabetic or hypertensive — but by trending us just a little bit more towards increased body fat.
Nowadays, it’s far more dramatic. We’re turning on the signal [of elevated UA] and it’s screaming day in and day out. What was (beneficial) through the lens of our ancestors is now through the lens of modern life a powerful manifestation of what we call an evolutionary/environmental mismatch. We can’t change our evolution, and we’re not going to be able to change our genome and therefore our physiology anytime soon. But we can change the other side of that relationship: the environment. We can stop triggering a survival mechanism that causes us to lay down more body fat, increases our blood sugar, raises our blood pressure, makes us insulin resistant, increases oxidative stress, decreases mitochondrial function…the list goes on.
HPC: Is there anything else you’d like our readers to know about UA?
DP: I’ll give you a few tidbits. It is really important that we maintain insulin sensitivity. Uric acid threatens insulin sensitivity [by] threatening nitric oxide (NO) production and function.
Why does this matter? NO does at least two important things. First, it targets the smooth muscle lining arteries. When NO is active, arteries relax. That improves blood supply. UA elevation threatens that, and therefore threatens blood supply to our most vital organs. It’s why there is such a significant increased risk of death from stroke in people with elevated UA.
“Elevated UA is upstream of all these other metabolic problems to which we’re so desperately responding.”
Here’s something else your readers may not know. The other thing NO is imperative for is the function of insulin. NO allows insulin to exit the artery and bind to its receptor on the cells, where it does its magic in terms of allowing glucose to enter the cells. There, the glucose can be utilized for energy or incorporated into glycogen, keeping glucose levels under control. So, the very fact that UA is elevated threatens to increase insulin resistance, through a heretofore unrecognized mechanism.
For our ancestors, it would have been a good thing to be insulin resistant and prediabetic. It allowed them to power their brains with sugar — with glucose. And it helped them avoid two things: starvation and predation. They could use their very clever brains to find food and avoid being eaten by another animal.
Importantly, when fructose is metabolized into UA, it consumes adenosine triphosphate (ATP). The first enzyme in the breakdown of fructose is fructokinase, which converts ATP to adenosine diphosphate (ADP). Then, that ADP becomes adenosine monophosphate (AMP).
One of the ways [the human body] deals with AMP is to activate AMP kinase (AMPK). When AMP is dealt with by AMPK, some very good things happen in the body. It helps us burn fat for energy, it helps us reduce fat storage, and importantly, it reduces gluconeogenesis — the production of glucose — in the liver.
We want to do our best to keep AMPK lit up, because it paves the way for health. How do we activate AMPK? Physical exercise. It’s one of the most powerful ways. It keeps us healthier, keeps our blood sugar in check, it lets us burn fat.
We can also activate AMPK by taking quercetin — that’s role number two for quercetin. We can activate AMPK by taking the drug metformin, which is used to keep blood sugar down in diabetics by reducing gluconeogenesis.
But it turns out that AMPK has an evil twin: AMP deaminase (AMPD). The activation of the evil twin does everything opposite. It increases fat storage, increases fat production, reduces fat utilization as an energy source, increases gluconeogenesis, and enhances mitochondrial dysfunction.
Our ancestors wouldn’t want to always have AMPK activated. They would want to have their AMPD activated as a survival mechanism. A bear getting ready to hibernate is all AMPD. The last thing that bear would want to do is activate its AMPK. It’s doing its very best to ratchet down its energy utilization. It’s doing what it can to make more body fat and lock it up. That’s why it is eating as much fructose as it can, and its UA levels go up.
Uric acid, when elevated, shuts down AMPK and activates the evil twin (AMPD). That’s powerful, it’s fundamental. I think over the years we’ve learned about ways to activate AMPK — and now we know what threatens it. It’s breathtaking.







