As a kid I grew up in doctors’ offices and labs. It’s not because I was sick. Rather my mother and father both worked there.
My mother was a microbiologist who applied those skills to live blood cell analysis and allergy testing, and then later worked in government microbiology labs where she researched models of tuberculosis dormancy. My father was a chemist who later studied herbal medicine, acupuncture, and then became a chiropractor.
My parents had a clinic where I played in the back as a young child, then worked the front desk when I got a little older. Medical practitioners, and practitioner-focused supplement products were part of my life from the beginning– I remember thinking all the Metagenics reps must have been models.
Quality-conscious medical practitioners can play a vital role in holding supplement companies accountable and driving them to truly make good on their quality and transparency promises.
Given this background it’s fitting that I now run Alkemist Labs, the supplement industry’s biggest little botanical testing lab. We focus on analytical testing to establish botanical identity, purity, and potency.

Because I have a particular affinity for healthcare practitioners, and the products that they recommend, I am grateful for the opportunity to share with you some of the essential things you need to know about botanical quality testing.
It’s important to understand that herbs are more complicated to test than vitamins, minerals, or other single component ingredients. Most of the latter have long-established specifications that they must meet, with “quality” indicated by a numeric value, such as how many grams of protein or vitamin A the ingredient contains.
While those are not necessarily easy analyses to perform, the definition of quality is very clear-cut. The ingredients need to meet some number. If they don’t, then they fail. It’s fairly well-defined.
Herbs are more complex.
To begin, let’s get philosophical. What is the definition of Ashwagandha root (Withania somnifera)? By simple labeling criteria, an ingredient marked “Ashwaghanda root” is not Ashwagandha leaf. A lot of Ashwagandha material on the market is labeled as “root” but it also contains Ashwaghanda leaf as a filler. Leaf isn’t bad but it isn’t the root, which is where most of the beneficial phytochemicals hang out. It may be the right plant, but it’s the wrong part. And by law, a label has to specifically disclose the contents.
“High quality” is a common value proposition, but it usually stays very shallow. The best brands in this industry are doing the work, and offering batch to batch lab reports to document the quality of their products.
Unfortunately, adulteration is rampant in the international market for botanical ingredients, as demand for herbal medicines rises and supplies vary year to year. It’s a problem that many people within the supplement industry are working diligently to remedy.

This also is why accurate identity testing for plant, and plant part, is crucial.
There are a variety of ways to test the identity of herbs, but not all of them are “fit for purpose.”
Using Ashwagandha as an example, when you are considering products for your dispensary, you need to know:
- A) Is it a sample with a genetic match to a control sample of the Ashwagandha plant?
- B) Does the sample give a light box response signal match with that of a library reference sample?
- C) Is it a sample with a specific quantity of Withanolides–a family of well-studied chemicals found in Ashwagandha and thought to be important in its biological activity and clinical efficacy?
- D) Do the microscopic cellular features match those of a verified reference material of the appropriate plant part, and not those of known adulterants, closely related species, or fillers?
- E) Does the chromatographic profile or “fingerprint” match that of a verified reference material of the appropriate plant part and not that of known adulterants or closely related species?
DNA Analysis: Regarding genetic testing, the reality is that DNA Analysis has its limits as a quality indicator. That’s because it can be easily tricked. DNA analysis only identifies the plant, not the specific plant part. Using our example of Ashwagandha, the root and the leaves will have the same genes.
So, DNA testing can tell us if we have the right plant, but not whether we have the right part, which is very important in terms of product quality.
DNA analysis is also not fit for purpose to test botanical extracts (tinctures, decoctions, etc), because typically there’s very little plant DNA remaining in a liquid extract.
Botanical supplement quality is dependent on physiological efficacy, and this is contingent on the specific phytochemicals in the herb. That said, it is important to remember that herbs are more than the sum of the chemical compounds they produce.
Light Box Methdology: Light box response testing, aka “Lab-In-A-Box,” is a new algorithm-based technology that deploys surface level analysis and promises the moon and the stars. Suffice to say, it does not always deliver.
High Performance Liquid Chromatography (HPLC): When it comes to analysis of biologically active compounds, HPLC is moving in the right direction, since most botanicals are sold based on claims to deliver specific families of phytochemicals. HPLC separates, identifies, and quantifies all the biochemical constituents of a given sample of plant material. This test will definitely tell you how much of each phytochemical constituent is in there.
Even with the FDA-mandated disclaimer of, ‘this product is not intended to treat, cure or prevent any illness,’ the truth is that from a practical viewpoint, botanical supplement quality is dependent on physiological efficacy, and this is contingent on the specific phytochemicals in the herb. Consequently, HPLC is an important quality measure.
That said, it is important to remember that herbs are more than the sum of the chemical compounds they produce. For example, the withanolides produced by Ashwagandha do not fully define the plant. Measuring a single or a few phytochemicals in a plant does not mean anything other than the presence or quantity of those phytochemicals.
A positive HPLC for caffeine does not mean you have good coffee; someone can add caffeine powder to inert sawdust, and it will give a nice strong caffeine signal on HPLC. But you would not want to drink it.
Microscopy: According to the FDA and other regulatory agencies worldwide, microscopy can confidently confirm the identity of a botanical ingredient in whole or powdered form. Microscopy looks at key cellular features of a given plant and compares it to verified reference materials, known adulterants, inert fillers, and flow agents.
Microscopy is an important factor in the quality equation, but it has a major limitation. As is the case with DNA testing, the material being tested must be in whole plant or powder form, and not an extract. Herbal extracts are like the brown liquid you get when you make coffee; they’re mostly void of the cellular connective tissue upon which microscopy relies.
A positive HPLC for caffeine does not mean you have good coffee; someone can add caffeine powder to inert sawdust, and it will give a nice strong caffeine signal on HPLC. But you would not want to drink it.
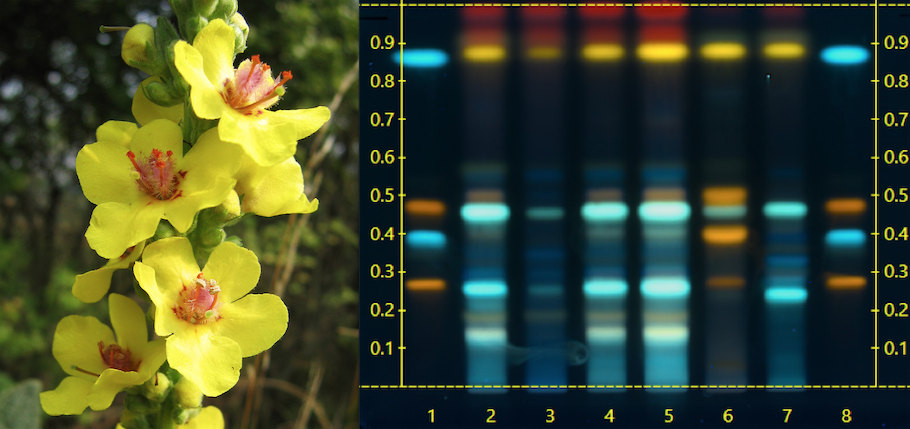
High-Performance Thin Layer Chromatography (HPTLC) Fingerprinting: This is my favorite approach to plant identity validation. It is elegant and nuanced, scientific, yet ‘eyeball-ometric.’ It provides both qualitative and quantitative information.
The process involves making an extract of the test sample using solvents, and then comparing the HPLC profile against that of the same type of extract made from verified reference materials. It is important to also compare the sample against the HPLC fingerprints of known adulterants.
So now that you have had a crash course in botanical identity testing, ask your supplement brands how they ensure that what is on their labels is what’s in their bottles. Better yet, ask them to just share their quality assurance data!
“Transparency” is a popular term these days. But it is not just another buzz word. I believe it is the next generation of brand differentiation.
Some brands spend millions of dollars each year testing their products internally and/or testing them with 3rd party labs like Alkemist Labs, but most fail to share that quality story.
“High quality” is a common value proposition, but it usually stays very shallow. The best brands in this industry are doing the work, and offering batch to batch lab reports to document the quality of their products. It’s an important advancement.
Quality-conscious medical practitioners can play a vital role in holding supplement companies accountable and driving them to truly make good on their quality and transparency promises.
END
Elan M. Sudberg is CEO of Alkemist Labs, one of the world’s leading contract testing laboratories. Based in Garden Grove, CA, Alkemist specializes in plant authentication, botanical ingredient identification and quantitative analytical services to the food & beverage, nutraceutical, and cosmeceutical industries. Alkemist is one of the only labs in the world that is ISO/IEC 17025:2017 Accredited for all plants. Elan holds a degree in chemistry from California State University Long Beach and has authored numerous journal articles on phytochemistry and analytical techniques for the natural products and nutraceutical industry. He is a board member of AHPA, as well as AHPA’s Education and Research on Botanicals Foundation.







