 Patients with joint hypermobility are very likely also suffering from a cluster of other chronic conditions that warrant close clinical attention.
Patients with joint hypermobility are very likely also suffering from a cluster of other chronic conditions that warrant close clinical attention.
While Ehlers Danlos Syndrome (EDS) and Marfan Syndrome may be the most dramatic and obvious examples, there are many other people with hypermobile joints that do not match these diagnoses. Scratch the surface in these cases, and you will often find a complex constellation of symptoms.
These include: Mast cell disorders, chronic fatigue/myalgic enchephalomyopathy, postural orthostatic tachycardia, circulatory and blood clotting abnormalities, chronic bowel disorders, post-traumatic stress, sex hormone and adrenal imbalances. Women with hypermobile joints show higher prevalence of gynecological conditions such as endometriosis and ovarian cysts, as well as autoimmune diseases.
In a sense, joint hypermobility could be considered a sentinel signal for other systemic diseases. What’s the connection?
A new theory forwarded by Dr. Sharon Meglathery posits that hypermobility and the associated systemic disorders are linked to an anomalous gene cluster on chromosome 6, known as RCCX.
Meglathery, a dually-boarded internist and psychiatrist in Tucson, AZ, began connecting the dots between seemingly disparate disorders and their genetic underpinnings when she herself developed “mast cell activation (MCAS), postural orthostatic tachycardia syndrome (POTS), raised intracranial pressure, chronic fatigue syndrome (CFS) and a host of other potentially disabling syndromes.”
Connecting Disparate Dots
She spent seven years “obsessed with a long list of seemingly connected, overlapping syndromes, gathering patient observations in my clinic and in the forums, scouring the scientific literature, dealing with my own illness and often having to experiment on myself.”
The puzzle pieces started to fit together when Meglathery read about RCCX–a gene cluster located in the middle of the major histocompatibility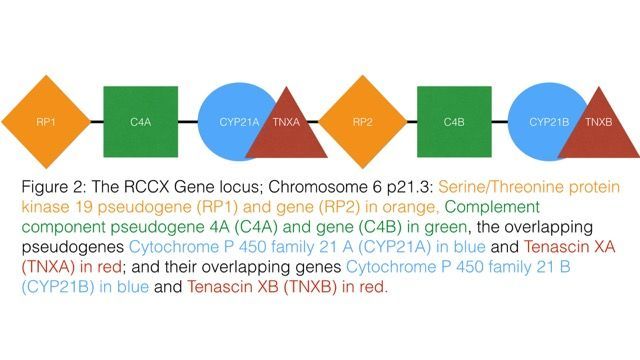 complex (MHC) region of chromosome 6. RCCX is actually comprised of four genes: TNXB (Tenascin-X), CYP21a2 (encodes for the enzyme 21-hydroxylase), C4 (complement C4), and RP1 (also known as STK19).
complex (MHC) region of chromosome 6. RCCX is actually comprised of four genes: TNXB (Tenascin-X), CYP21a2 (encodes for the enzyme 21-hydroxylase), C4 (complement C4), and RP1 (also known as STK19).
The RCCX sequence is known as a “copy number variation” (CNV), meaning that the sequence produces copies of itself (referred to as monomodular, bimodular and trimodular forms). CNVs represent between 5% and 10% of all human genetic variations, and they are highly unstable. The best-known CNVs are those linked to Huntington’s disease and Fragile-X syndrome.
According to research, the RCCX cluster behaves as one unit rather than as four separate genes. When one gene is mutated, the other three are also affected.
What’s interesting is that the genes that comprise RCCX each confer very different functions across multiple physiological systems. This is the key to understanding how such seemingly unrelated conditions as orthostatic tachycardia, joint hypermobility, and ovarian cysts might in fact reflect the same genetic anomaly.
From a clinical perspective, if one sees a patient with joint hypermobility as well as a complex symptom presentation, there is a high probability of RCCX cluster abnormalities. By studying the effects of these genes we can learn more about who may be affected, and how to develop effective treatment strategies.
TNXB & Extracellular Matrix
The extracellular matrix (ECM) is not something that gets much press, yet it plays an integral role in cell-to-cell communication, cell cycle functions, and regulation of growth factors. When cells lose their connection to the ECM, they typically undergo apoptosis.
Tenascin-X (TNXB) is the major glycoprotein in the ECM, and it plays a critical role in collagen architecture and cell adhesion, as well as in the proper utilization of growth factors such as transforming growth factor beta (TGFß) and vascular endothelial growth factor (VEGF). One of the four components in the RCCX cluster is the gene that codes for TNXB. Deficiency of TNXB is one major cause of joint hypermobility.
TNXB haploinsufficiency–a mutation that results in an insufficient gene product or enzyme–results in a very rare form of EDS characterized by joint hypermobility. The RCCX theory contends that mild mutation of TNXB can produce a hypermobile phenotype without haploinsufficiency, and a 2016 study supports this (Chen W, et al. Hum Mutat. 2016; 37(9): 893-7)
Insufficient production of TNXB leads to collagen deficiency, and disrupts the normal response to growth factors, especially TGFß and VEGF. This ultimately results in immune imbalances, because TGFß plays a critical role in balancing TH1 and TH2 immunity. TNXB insufficiency promotes inflammatory TH17 signals, which create a breeding ground for autoimmune disease.
Among women with joint hypermobility, endometriosis is common. It’s interesting to note that endometrial lesions typically contain high amounts of TGFß. It may be possible to prevent and treat endometriosis by modulating the extracellular matrix and normalizing the tissue response to TGFß.
When TNXB levels are low the circulatory system can suffer as well. This is because low TNXB alters VEGF, which is essential for blood vessel formation, oxygen delivery to tissues, and recovery from exercise. Individuals with the suspected RCCX anomalies often experience bruising and circulatory problems, as well as intolerance to exercise.
It is interesting to note that many people report positive circulatory and stamina effects from taking a special form of copper, marketed as Mitosynergy. Copper is known to potentiate VEGF and its myriad effects. Copper is also involved in the formation of collagen through an enzyme known as Lysyl oxidase.
Additional protocols which may effectively modulate the ECM include polysaccharides derived from aloe vera and other plants (possibly Gotu kola), collagen peptides, glycosaminoglycans (such as chondroitin sulfate and glucosamine), as well as modulating water and electrolyte balance.
CYP21a2: Adrenals & Sex Hormones
The gene that sits next to TNXB on the RCCX cluster is CYP21a2. It converts progesterone into cortisol and aldosterone, respectively. Haploinsufficiency of CYP21a2 is known to cause congenital adrenal hyperplasia (CAH). However, mild mutations of CYP21a2 have been known for decades, and can lead to numerous complications without showing up as CAH.
CYP21a2 insufficiency leads to a bottleneck of progesterone, which then gets shunted into androstenedione. The excess results in either elevated testosterone and androsterone, or it aromatizes into estrone, E1.
It’s for this reason that mild mutations of CYP21a2 cause high androgens in both men and women, and associated symptoms of ovarian cysts, PCOS, hirsutism, amenorrhea and acne. All are common conditions among people with joint hypermobility.
Additionally, mild mutations of CYP21a2 may also reduce cortisol and aldosterone. It’s for this reason that people with the RCCX phenotype often show erratic serum electrolytes, especially sodium, potassium, and chloride. A loss of sodium through the urine due to insufficient aldosterone will adversely effect on the electrolyte balance in the ECM. It also lowers blood pressure (primarily causing hypotension) and alters blood clotting (due to aldosterone’s little known role of clotting).
The reduced capacity to manufacture sufficient cortisol at the proper times leads to feedback mechanisms in the brain generating too much ACTH and/or CRH, as well as a reduced capacity to cope with stress.
As part of her RCCX theory, Dr. Meglathery contends that CYP21a2 is responsible for the myriad psychiatric complications among people with severe forms of RCCX abnormality.
 The developing brain, exposed to high androgens and deranged cortisol in utero, has a greater susceptibility to PTSD, and disordered emotional processing in the limbic system. On the other hand, RCCX genotypes may also lead towards brilliance and a high intellectual capacity, but at the expense of emotional processing and possible psychiatric illness.
The developing brain, exposed to high androgens and deranged cortisol in utero, has a greater susceptibility to PTSD, and disordered emotional processing in the limbic system. On the other hand, RCCX genotypes may also lead towards brilliance and a high intellectual capacity, but at the expense of emotional processing and possible psychiatric illness.
Dr. Meglathery believes the RCCX theory holds tremendous promise for elucidating a central genetic component in psychiatric disorders.
A single case study published in 2012 found that 800mg of Ashwagandha root (Withania somnifera) –a common plant medicine in Ayurveda–significantly attenuated multiple steroid hormones that were out of balance in a patient with mild CYP21a2 deficiency. This included modulation of 17-OH progesterone, 18-OH hydroxycorticosterone, 17-OH pregnenolone, corticosterone and 11-deoxycortisol (Kalani A, et al. BMJ Case Reports 2012).
Berberine, a plant alkaloid derived from Barberry, may also help by modulating the conversion of progesterone to androstenedione, as well as VEGF activities.
Complement C4 & Immune System
The third gene on the RCCX cluster codes for complement C4, a critical immune protein that plays important roles in the immune system, and in dendritic pruning. C4 is split off into subtypes C4a and C4b, both of which reside on the RCCX cluster. Complement C4 and C4a are involved in the immunological responses to Lyme disease and mold toxicity.
The complement immune system is a complex series of cascades, divided into 3 branches: the Lectin pathway, the Classical pathway, and the Alternative pathway. Mild complement C4 deficiency is considered to be one of the most common types of immune deficiencies.
In the Lectin pathway, C4 is handed off to polysaccharides and collagen (called Ficolin) for the purpose of creating adhesive bonds to viruses, bacteria, and fungi.
Deficiency of C4 is strongly linked to numerous types of autoimmune diseases, especially Lupus erythematosus (with deficiency of C4 found in as many as 75% of cases), Type 1 diabetes, multiple sclerosis and rheumatoid arthritis. The complement immune system plays important roles in the activation and inactivation of mast cells and histamine release.
It is common for people with RCCX anomalies to exhibit mast cell disorders, as well as problems modulating histamine response.
Because C4 regulates dendritic pruning, it is involved in neural health and development. It is noteworthy that C4 deficiency is linked to schizophrenia and mental illness.
An interesting phenomenon within the RCCX cluster is the presence of an endogenous retrovirus, known as HERV-K, situated in the C4 gene. Some studies have demonstrated that HERV-K retroviruses are capable of causing copy number variations (CNVs) as well as deletions of nearby genes.
This is potentially very significant because RCCX is comprised of CNVs.
The human genome actually contains many retroviruses. Most are inactive; however recent studies have identified activated HERV-K in connection with amyotrophic lateral sclerosis (ALS). Some researchers believe that HERV-K confers a protective effect against other viruses, while others argue that HERV-K activation is pathogenic and linked with numerous diseases.
Nevertheless, the presence of an endogenous retrovirus within the C4 gene should set off alarms for researchers and geneticists interested in understanding the implications of RCCX, and the cluster’s genomic instabilities.
Polysaccharides like mannose, polymannan and others may provide a significant benefit for mild C4 deficiency. Anecdotal evidence supports this contention, though there are no studies.
In a sense, Sharon Meglathery’s RCCX hypothesis provides a “unified field theory” for explaining the co-occurrence of diverse and difficult-to-manage medical conditions. If Meglathery’s postulates are validated by future research, they have potential to open entirely new treatment options for some very vexing disorders.
The next phase of RCCX research involves sequencing the cluster in a larger population. This is no small task. Presently there are only a few labs in the world capable of sequencing RCCX. Additionally, obtaining various types of population data, clinical observations, and lab biomarkers are all critical aspects of moving this theory forward.
Clinicians interested in learning more about RCCX and its relationship to real-world symptom patterns are encouraged to read Meglathery’s detailed summaries of the theory and the background research supporting it.
To the best of our knowledge, Meglathery and her research partner, endocrinologist Karen Herbst, MD, are the only group looking at RCCX in the context of clinical care for people with chronic illness.
They have established a research organization called The RCCX Project, through which they will be gathering “a large amount of family and clinical data from people with chronic illnesses (CFS, chronic Lyme, FM, etc), hypermobility, the CAPS profile, CYP21a1 mutations, and subcutaneous adipose tissue disorders and will compare them with normal controls.” Meglathery and Herbst add that they are actively seeking geneticists and other scientific collaborators.
In light of what’s already known, it makes clinical sense to ask all patients about joint hypermobility, and to pay special attention to those who have signs of the condition. Odds are high that they will also have signs and symptoms of other chronic conditions. If you ask, you might also find that there are positive family histories for these disorders.
END
Michael McEvoy, FDN, CNC, CMTA is a clinical nutritionist who is the founder and director of clinical development at Metabolic Healing, a website and online education platform dedicated to facilitating the organic evolution of all people toward the highest level of health and well-being.
In addition to functioning as a clinician and writer, Michael is a teacher, educator and systems creator of diverse health-related and functional medicine curricula. Through unique educational and teaching endeavors, Michael’s objective is to assemble a network of the world’s top clinicians to meet the demands and challenges of 21st century functional medicine, and to implement the analytical tools and frameworks required.
He also maintains a clinical consulting practice based in Santa Cruz, CA.







