 Am I infected? Am I infectious? Am I immune?
Am I infected? Am I infectious? Am I immune?
These are simple, reasonable questions that millions of people—practitioners and patients alike—are asking every day, all over the world.
It would be nice if there were simple answers. There aren’t.
Tests to identify SARS Cov-2, and immune responses to it, are vital both for clinical decision-making and public health policy. But even with wide use of the most sophisticated tests, there would still be much variability and many unanswered questions. Testing is not foolproof nor failsafe. And it is definitely not simple.
“Having a positive viral RNA PCR is not the same as being infective. Having antibodies to the virus is not the same as immunity,” said Patrick Hanaway, MD, head of the Institute for Functional Medicine’s COVID-19 Task Force.
In a webinar on the state of COVID testing in late April, Dr. Hanaway and Dr. Helen Messier offered practical guidance on how best to use the available tests.
“It is going to be frustrating at times, when you don’t have a complete answer, when there’s uncertainty around a test result. We don’t have 100% certainty, and we need to embrace that,” added Dr. Messier, an immunologist and family physician at Altum Health in San Jose, CA. “Even though we’ve learned a lot about the virus, about testing, and about the disease in a very short time, we have a lot left to learn.”
When assessing a patient who has likely been exposed, there are four key questions: Are there symptoms? If so, when did they begin? Is there detectable evidence of the virus? Is there an antibody response?
Identifying Viral Exposure
Currently, there are two main ways to determine if someone has the virus: reverse transcription polymerase chain reaction (RT-PCR) to detect viral RNA in sputum, saliva, or nasopharyngeal secretions; or viral culture of these secretions. The two methods give very different information.
RNA tests confirm that someone has been exposed, but they cannot determine if there is active viral shedding—that is, whether the person is infective. That requires a viral culture, which is seldom done outside research settings.
“Just because we see positive viral RNA on a swab does not mean that they are shedding actual infectious viral particles. If you take the swab and you can grow the virus in culture you know that it is infectious. But the viral RNA is what most people test, because setting up biocontainment labs where you can do viral cultures is very difficult. Viral RNA is much easier to study, but it is not definitive,” Messier explained.
Understand the Timeline
“The value of the test is in the context of the test,” she stressed.
The key is to understand the timeline of the infection and the body’s responses.
She and her IFM colleagues have read hundreds of papers on the immunology of SARS CoV-2, synthesizing the findings into a comprehensive graph that plots the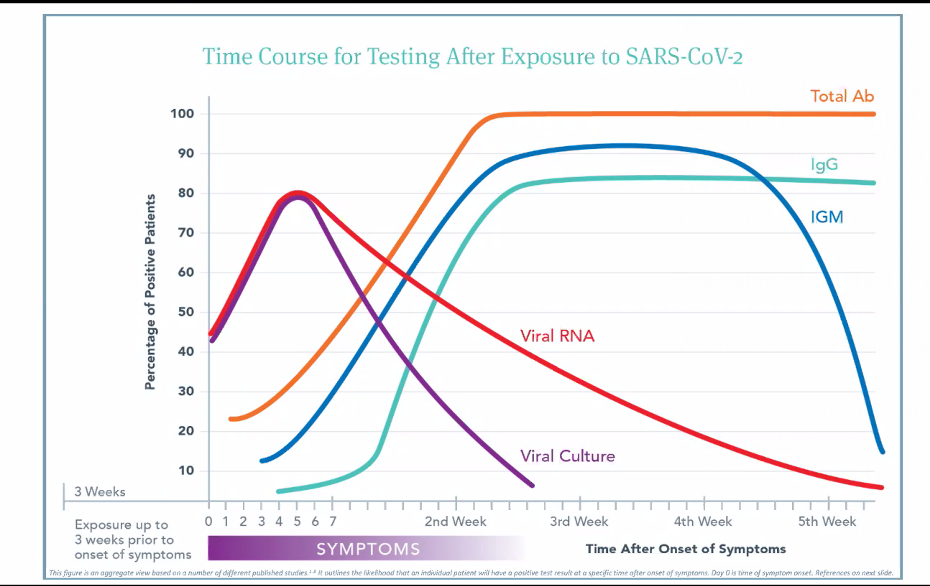 likelihood of a positive test result against the passage of time following symptom onset.
likelihood of a positive test result against the passage of time following symptom onset.
The main point is that RNA tests and serological tests give variable results depending on when you do them.
“The span of time between exposure and development of symptoms can be very different in different people. It is anywhere from 2 days to 3 weeks. Some people will continue to be asymptomatic indefinitely. However long it is, there’s a time period during which people will likely be shedding the virus even though they don’t have symptoms, and we wouldn’t even think to test them because they’re asymptomatic,” Messier said.
Immunologically SARS CoV-2 follows a fairly predictable course, though the clinical manifestations vary widely from patient to patient.
Viral titers usually hit their peak 4-6 days after symptom onset, and then drop fairly quickly. “There’s a window in which you can test positive and capture that virus. But it is easy to miss that window,” said Dr. Messier. This is a main reason why the RNA PCR tests have a false-negative rate upward of 30%.
“By 3rd week post-symptoms, it is much less likely that someone will show viral titers. You’ll see a lot more negative RNA tests, even though people have the virus.” But it is also true that viral RNA can be present in people who are no longer symptomatic.
In other words, a positive RNA test does not mean someone is sick, or will get sick. Nor does it mean that he or she is infectious. All it indicates is exposure.
For those who do get sick, the symptomatic period is also variable. In some, it is just a few days, in others it can be up to 3-4 weeks.
One of the biggest clinical challenges is that many are not sure when they first had COVID symptoms. When they’re really sick—the point at which they become “eligible” for testing–they may not be in any condition to answer such questions clearly. Without knowing anything about symptom onset, it is difficult to put test results in context.
Viral shedding—the hallmark of infectivity– begins anywhere from 3 to 21 days following exposure. Viral particles can be detected in the nasopharynx, oropharynx, saliva, and sputum, and shedding may—or may not—go on for up to 21 days after symptom resolution. In general, it seems that shedding declines roughly 14 days after symptom onset. But again, many patients don’t know when the illness started.
Viral RNA may be detectable long after viral shedding has stopped. But beyond indicating that someone has been exposed, the clinical and public health significance of a positive RNA test after symptoms abate is largely unknown.
Assessing Immune Response
Antibody testing is the other main factor in the assessment equation. Like viral detection, this is also highly variable and time-dependent.
“Based on initial data, it looks like it (SARS-CoV2) follows the general course (for viral infections), which is that IgM, which is a more non-specific antibody class, arises first and then over time, the more specific antibodies of the IgM class will class-switch over to IgG, which is a more specific antibody. IgG levels rise later,” Messier explained.
The total antibody level—IgG, IgM, and IgA together—tends to rise first, during the first and second weeks after initial symptoms. This largely reflects the IgM response. It typically peaks between the second and third post-symptom week. Median antibody production is at about Day 15 post-symptom onset.
As the total antibody and IgM curves reach their summit, IgG production ramps up. By the 4th week, IgM drops off, but IgG remains at peak level. Presence of IgG pretty much confirms exposure. This is the general pattern, based on the best available data from observation of hospitalized patients.
The timing of these phases has significance for clinical testing, said Dr. Messier.
“You have to wait at least two weeks, sometimes up to three weeks to really be able to trust that someone has an IgG response. If you test too early, you may miss it because the patient has not mounted that IgG antibody response yet.”
What about Secretory IgA and the mucosal immune system? What does that tell about the state of the immune system?
“We know there’s IgA made against SARS-CoV-2, but I don’t know that there are any studies showing that if you have huge amounts of secretory IgA it means your immune system is able to fend off this virus. Secretory IgA fights off a lot of things. If you have other infections—oral infections for example–the secretory IgA levels may reflect the body’s efforts to block those other things.”
An IgA surge indicates that the immune system is active, but it is non-specific for SARS-CoV-2.
 Dr. Messier pointed out that there’s a point on the timeline—usually around Day 7-10—where all the lines on the graph meet and cross.
Dr. Messier pointed out that there’s a point on the timeline—usually around Day 7-10—where all the lines on the graph meet and cross.
“Viral RNA titers are dropping, but antibody responses are rising. If you test a patient at that point in time, they could be negative to everything even though we know there was a viral exposure, and they’re developing antibodies. The tests might all be negative just because of the timing.” But there’s also a possibility they could be positive for everything.”
Also bear in mind that immunocompromised patients—and many severe COVID patients fit that description–might not show any antibody responses. A negative antibody test does not rule out viral infection; antibodies reflect the state of the immune system, not presence or absence of the virus.
Antibodies & “Immunity”
Do any of the known antibody responses confer actual immunity? That is a big, largely unanswered question at this point.
Dr. Hanaway explained that testing labs have developed assays to detect antibodies for a wide range of potentially antigenic coronavirus proteins—the spike proteins that give these viruses their characteristic appearance, proteins on the viral envelope, proteins on the membrane. The clinical significance of having antibodies to any of these is not always clear.
“What you are measuring—whether it’s the spike protein or the membrane protein or the envelop protein—is going to affect the sensitivity and specificity of the assay. The M protein is specific and seems to be conserved in this virus over time, the S protein may change as the virus morphs. You might get a false negative simply because the spike protein varied,” he said.
Some antibodies–like antibodies to the spike protein–may be “neutralizing,” meaning that they neutralize the virulence of SARS-CoV-2. Others are not.
“Neutralizing antibodies prevent the virus from entering cells. The virus cannot enter a cell that’s covered with neutralizing antibodies,” Dr. Messier explained. “Other antibodies are binding antibodies that don’t block the viral entry into cells, but they recruit the T-cells to destroy the virus.”
In any given case, the patient’s antibody response might—or might not—be able to prevent recurrence of disease. “We are not able to assess immunity directly. But we’re making proxies of it,” Dr. Hanaway noted.
The predictive value of antibody tests for coronavirus—or any pathogen for that matter—also depends in part on overall prevalence of the pathogen in the general population, added Dr. Messier.
“If the test is 95% specific, and there’s 1% of the population that has the antibodies, you will be wrong five times more frequently than you’re right. If the prevalence is 25%, and the specificity is the same at 95%, you will be right five times more than you are wrong.”
On a total population basis, the prevalence of SARS-CoV-2 is still fairly low, meaning that from a statistical perspective there are likely to be a lot of wrong answers.
Clinical Considerations
Who to test: If someone has the “classic” COVID symptom pattern, test. And then test again, said Dr. Hanaway. “Any symptoms should be considered COVID unless proven otherwise. We have to err on the side of caution, but perform the RNA test to confirm the infection. If the test comes back negative, wait 7 days or more, then do antibody tests.
Should you routinely test asymptomatic but at-risk patients? Maybe. If someone’s had a likely recent exposure, but needs to be out in the world (working at an “essential” job, for example) it is wise to do a viral RNA test. If the patient is sheltering at home, not sick, and not likely to expose others, the test may not be necessary.
If a patient had close and frequent contact with someone who has been sick with COVID, then RNA testing is strongly recommended. “But even if that test comes back negative, we still advise quarantine for 2 weeks, because we know the false negative rate is high. After that two-week period, it makes good sense to do some serologic tests to see if the patient generated antibodies.”
Obtaining test samples: Talking about testing is easy. Getting good samples is not.
Viral RNA is present in sputum, saliva, nasopharyngeal secretions, and feces. While many different testing methods claim high sensitivity and specificity, in reality most have only been validated for one or two sample types–usually nasopharyngeal swab samples–but not the others.
According to Dr. Messier, nasopharyngeal testing “has very good sensitivity. But is really awful. It’s not a great way to sample. The patient’s going to start coughing,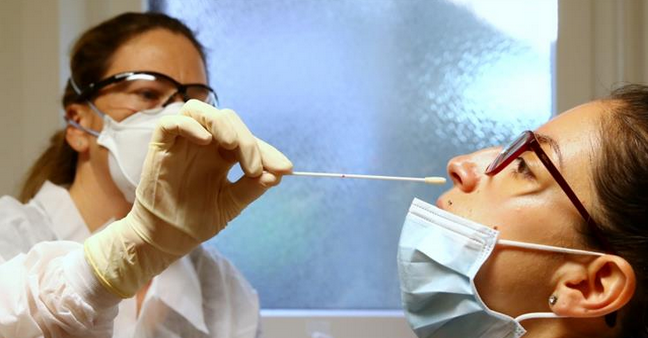 spraying droplets everywhere, spreading the virus. It’s important for the person doing the sample to wear proper protective equipment.”
spraying droplets everywhere, spreading the virus. It’s important for the person doing the sample to wear proper protective equipment.”
There’s an added problem: many clinics are running out of swabs and unable to order more. “Some people have begun 3-D printing them,” she said.
Sputum sampling is easier, but it will only provide reasonably accurate results if you can get a first morning sample of mucus from the lungs and the back of the throat. Saliva testing is more practical, and is being widely used in some countries. But saliva tests have not been fully validated.
Stool testing is possible in principle. It is being used at the public health level to assess viral loads in sewage in various communities, said Dr. Hanaway. Clinically, it is not yet relevant. “We know viral RNA is present in stool but they’ve never been able to culture virus from stool. All it really tells you is that someone has been exposed.”
Point-of-Care and Home Test Kits: Widespread testing requires easily accessible tests. That has been problematic in most countries, including the US. The biotech and diagnostic testing industries are in overdrive right now, developing and releasing new point-of-care tests, and DIY home test kits, at a rapid clip.
“To stop the virus spreading…will take a massive effort to scale up the production of easy-to-use POC tests and then to deploy them widely,” wrote Cormac Sheridan, in a recent article in Nature Biotechnology detailing some of the emerging methods. Sheridan points out, however, that quickie tests to detect antibody responses have been faster to emerge than simple tests to detect the virus itself.
Hundreds of new COVID-related tests have hit the market in the last few weeks, prompting the FDA in May to impose a new rule requiring test-makers to apply for emergency-use authorization and FDA review when launching new tests. Previously, the FDA allowed companies to do their own validation testing and to market COVID tests without prior FDA evaluation of sensitivity and specificity.
Many new tests are modeled on home pregnancy tests. They require just two drops of blood, and promise low cost, convenience, and privacy–appealing attributes. But the validity of these tests is questionable.
Danish reserchers assessed nine commercially available test systems—three ELISA-based and the rest using lateral flow technology. They used serum samples that they knew with certainty were either positive or negative for SARS-CoV-2.
“The results were all over the place,” said Dr. Messer. “There were only three that came even close to getting it right. There were a lot of false positives. And with convalescent plasma, it was like rolling the dice. The test that gave the fewest false positives, also missed about 10% the true positives.”
According to the IFM reviewers, the jury is definitely still out on the role of these tests.
Cross-Reactivity & Test Validation: There are at least four other types of coronavirus circulating in the general population, besides SARS-CoV-2. From both a clinical and public health perspective, they can cloud the testing picture.
The widely used RT-PCR methods have been validated against the other four coronavirus types, as well as other common viruses. But many of the other types of tests—especially the antibody tests—have not.
When a test comes back positive, you want to assume this reflects a recent or current infection with SARS-CoV-2. But unless you are certain that the specific method has been assessed for cross-reactivity, you cannot rule out other coronavirus strains.
“Maybe your patient had a cold recently. Owing to this cross-reactivity, that may show up as a false positive for SARS-CoV-2,” Dr. Messier said.
She challenged the “100% specificity” claims made by many test marketers. “I’ve not seen any lab—and I have looked at quite a lot—that has done their validation studies looking at that cross-reactivity with the other coronaviruses. Many will look for other respiratory viruses, but they don’t test for those other coronaviruses.”
Practitioners have a big role to play in driving improvements in test validity. The IFM strongly encourages all clinicians who do COVID testing to insist that labs provide complete analytical and clinical validation data. The organization has created a standard question set for communicating with labs (see Evaluating COVID Serology Tests).
When is someone “safe”? How do we know for sure that someone who has tested positive for SARS-CoV2 is non-infectious and safe to go back into life? We don’t.
But Dr. Messier said there are basic minimum criteria: “We don’t want to see any more viral RNA in respiratory droplets. If you see no viral RNA, and you also have antibodies, you’re probably safe to go back to work. But the viral RNA test should be done twice because the false negative rate is high.”
Testing for SARS-CoV-2 and COVID is a rapidly evolving field. New studies and new testing systems are emerging nearly every day. We may never reach a point of 100% diagnostic certainty. But wider testing, good clinical judgment, and honest communication will help us move in the right direction.
END







