The last decade’s worth of microbiome research has taught us that much of our “human” physiology is actually regulated by microorganisms in our digestive tracts. It turns out that some of these organisms need not even be alive to exert their influence.
Welcome to the world of paraprobiotics—an emerging category of health products containing heat-killed organisms that none the less retain their ability to beneficially interact with the human immune system.
Researchers at Kirin, the Japanese brewing company, discovered particular strains of heat-killed Lactococcus lactis and Lacticaseibacillus paracasei that are able to activate plasmacytoid dendritic cells (pDC) in the Peyer’s patch regions of the gut mucosa, leading to activation of natural killer cells, T cells, and B cells, while also boosting production of regulatory cytokines.

The advantage of heat-killed organisms over live bacteria is that the former are stable at room temperature. They do not need to be refrigerated, they’re easier to formulate into a wide variety of products, and they do not need to colonize the user’s GI tract to be beneficial, explained Danielle Citrolo, RD, VP of Scientific & Regulatory Affairs for Kyowa Hakko, a subsidiary of Kirin specializing in nutritional ingredients.
In a webinar on the clinical uses of paraprobiotics, Citrolo defined them as “non-viable microbial cells that confer health benefits.”
She added that paraprobiotics are comparable in many ways to live probiotics. But since these organisms are no longer living, there are no concerns about temperature stability or viability.
Basic and clinical research shows that paraprobiotics have immunomodulatory and anti-inflammatory effects. Like many probiotics, they can improve gut barrier function, ease constipation, reduce symptoms of stress, and in some cases control Helicobacter pylori (Siciliano RA, et al. Nutrients. 2021). But the mechanisms by which they do this are quite different.
Targeting Peyer’s Patches
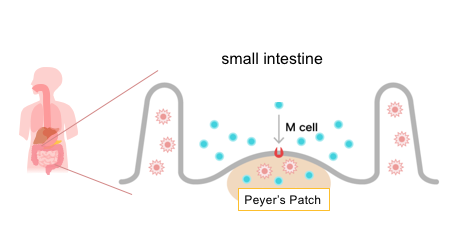
Probiotics typically exert their diverse physiological effects by colonizing the large intestine. In contrast, paraprobiotics act in the small intestine, through interactions with cells in the Peyer’s patches. These regions of the ileum are a part of the complex network of gut associated lymphoid tissues (GALT). The Peyer’s patches are one of the most important components of the immune surveillance system.
Microfold cells (M cells) within the Peyer’s patches are the initial point of contact between paraprobiotics and the immune system; these cells transport the prokaryotic cells into the lamina propria where they are introduced to the various lymphocytes.
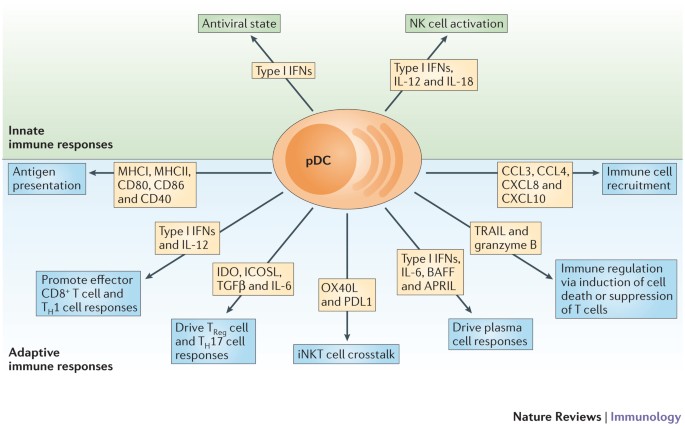
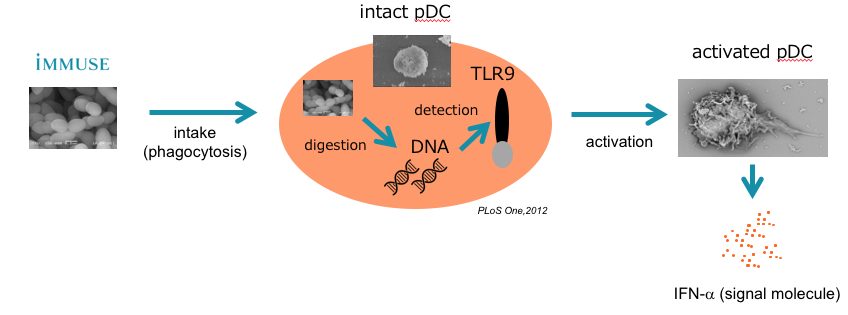
The L. lactis paraprobiotic, one of the two developed by Kirin, activates the plasmacytoid dendritic cells (pDCs) within the lamina propria. These cells are considered to be the “quarterbacks” of the cellular immune system.
After pDC cells phagocytize the L. lactis cells, DNA from the latter binds to TLR9 receptors inside the pDC cells. Once activated, the pDCs secrete interferon-a, a key signaling molecule that primes and directs the NK cells, T cells, and B cells. The pDCs are the body’s major producer of IFN-a.
Activating the “Right” Cells
According to Citrolo, the pDCs are the “right” immune cells to stimulate for broad range immune system support. They are capable of detecting most pathogens and they regulate and coordinate the other immune cells.
“This is really a broad activation. By activating all of the cells, it’s orchestrating a more broad-range immune response.”
Most probiotics, as well as other immune system stimulating ingredients such as mushroom b-glucans, act directly on the natural killer cells. Very few are able to activate the pDC cells. Consequently, these products do not provide the more comprehensive immune system priming that is possible with the paraprobiotics.
In a study of 51 healthy university athletes, the subgroup that was randomized to take the L. lactis paraprobiotic daily for two weeks showed a roughly 30% increases in pDC activation, as indicated by increased expression of the CD86 marker. There were no such changes in the placebo group (Komano Y, et al. J Int Soc Sports Nutr. 2018).
There are now 11 human clinical studies supporting the use of Kyowa Hakko’s patented L. lactis strain, which is marketed as a dietary supplement and food ingredient called ImmuseTM. The typical dose is 50 mg per day (roughly 100 billion heat-killed cells). It comes in powder form and mixes readily with water. It has a neutral taste, and can be easily incorporated into various supplement formulas or food mixtures.
Minimizing Cold & Flu
Kirin researchers studied a cohort of 213 healthy subjects between the ages of 30 and 50 years, who were randomized to take Immuse or a placebo daily for 10 weeks. They found that the incidence of clinically-diagnosed cold and flu was lower in Immuse group (7 vs 14 cases). Among the Immuse patients who did have colds or flu episodes, the burden of cough and fever was significantly lower (Sugimura T, et al. Br J Nutr. 2015).
Investigators at Tokai Unversity in Kanagawa, Japan studied 397 healthy volunteers, aged 18-39 years, randomized to either placebo or Immuse for 12 weeks. They recorded a significant reduction in the number of incident days of cough and sore throat among the subjects taking Immuse, concurrent with a marked increase in expression of IFN-a.
“These data suggest that the intake of heat-killed JCM5805 (the strain in Immuse) stimulated pDC-related anti-viral immunity to mitigate the incidence of common cold and influenza symptoms,” the Tokai researchers concluded (Shibata T, et al. J Funct Food 2016.)
The paraprobiotic has potential benefits for children. Kiyomi Sakata, of the Department of Hygeine and Preventive Medicine, Iwate Medical University, led a community-based intervention study to document the impact of daily paraprobiotic supplementation on rates of influenza.
They compared incidence of flu among elementary and junior high school students over a 3-month winter in two towns in the Iwate prefecture. The towns were similar in their demographic and socioeconomic profiles. The only difference is that in one town, the schools provided yogurt containing the Immuse paraprobiotic thrice weekly for three months, while the other town did not.
The peak flu prevalence was notably lower in the town where the kids got the paraprobiotic yogurt (5.2% versus 7.3%, taking the two age groups together). “JCM5805 yogurt intake three times a week over a 3-month period reduced the incidence rates of influenza by approximately two-thirds,” Sakata and colleagues report (Sakata K, et al. Health. 2017).
At the other end of the age spectrum, there’s also evidence that daily supplementation with a heat-killed L. paracasei probiotic can bolster antibody responses to influenza vaccines among people over the age of 85 years (Maruyama M, et al. Int J Food Sci Nutr. 2017).
Beyond colds and flu, there are clinical studies showing that taking paraprobiotics can mitigate symptoms of atopic dermatitis, dust mite-induced allergic rhinitis, and pollen allergies.
The Gut-Eye Axis?
One of the most intriguing aspects of the paraprobiotics story is the finding that taking heat-killed probiotic bacteria has effects on the eyes.
The L. paracasei KW31120 strain, developed by Kyowa Hakko as an ingredient called Eyemuse, will induce secretion of interleukin-10 (IL-10) after it binds to and activates the M2 macrophages in the small intestine. IL-10, an important regulatory cytokine, enters the bloodstream and reaches the eyes where it can down-regulate ocular inflammation.
There are several in vitro studies to suggest that paraprobiotic stimulation of IL-10 secretion can reduce the retinal damage caused by persistent blue light exposure.
More recently, a study of 62 healthy human volunteers showed that mitigate symptoms of eye strain caused by prolonged periods in front of computer monitors and other visual display screens (Morita Y, et al. Nutrients. 2018).
This is an emerging line of research, one that suggests the possibility of a “gut-eye axis.” It opens a new chapter in the evolving saga of the interaction between microbes and humans.
END







